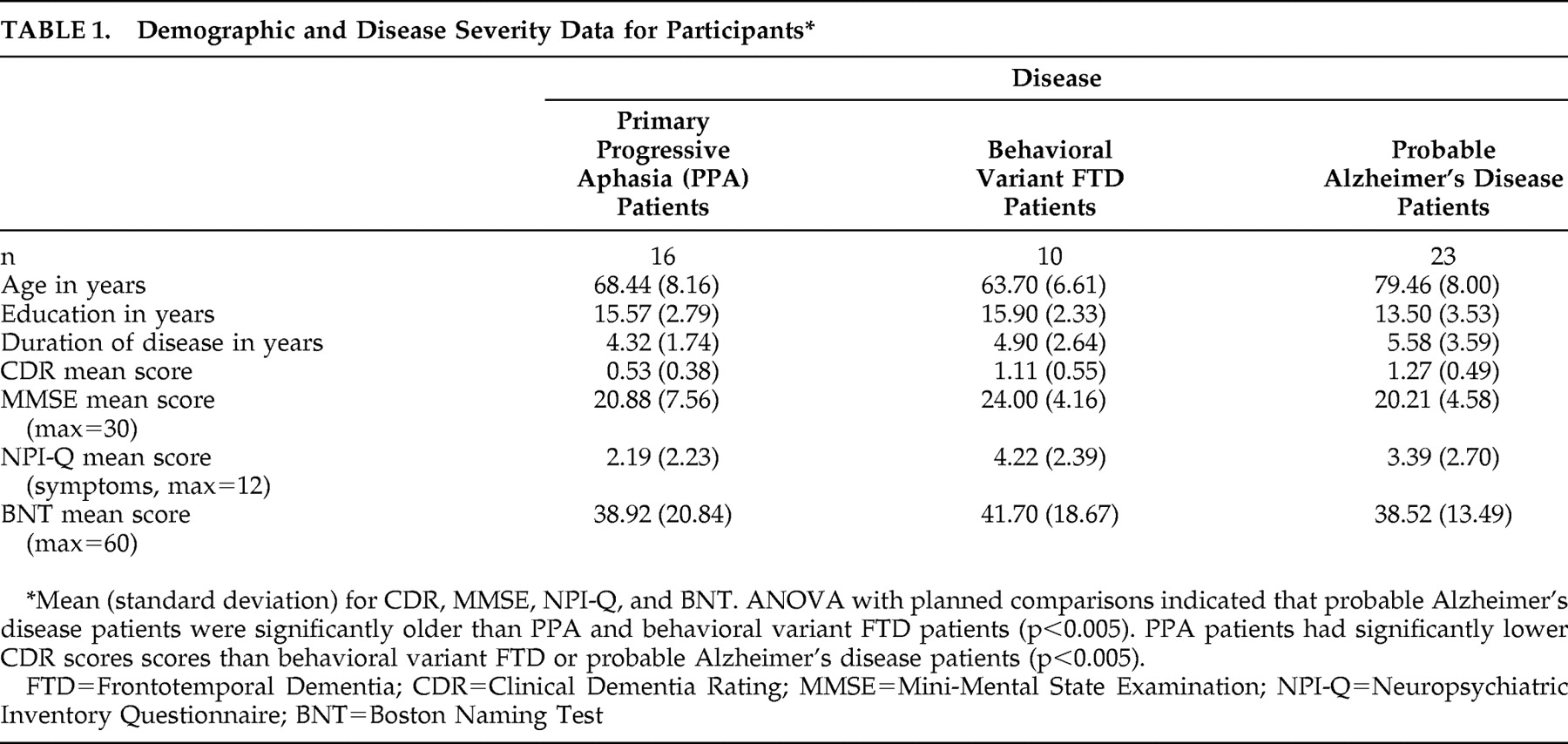
A nosognosia, or loss of insight for symptoms, has been reported in various neurological disorders
1 including dementia.
2,
3 An intriguing dichotomy in insight exists between two variants of dementia caused by frontotemporal lobar degeneration (FTLD), the behavioral variant frontotemporal dementia (FTD), and the language variant (primary progressive aphasia or PPA). A core diagnostic criterion for behavioral variant FTD is the early loss of insight, which is defined as “a lack of awareness of mental symptoms evidenced by frank denial of symptoms or unconcern about the social, occupational, and financial consequences of mental failure,”
4 thus combining both a frank loss of insight (anosognosia) with a lack of concern about acknowledged symptoms (anosodiaphoria), although studies indicate that some behavioral variant FTD patients show partially intact insight early in their disease course.
5 In PPA, studies indicate that insight is spared early in the disease but diminishes over time.
6,
7 With increasing disease duration, the symptom profiles of these two related dementias become less distinct: symptoms of aphasia can emerge later in the course of behavioral variant FTD,
8 and PPA patients often become behaviorally more similar to behavioral variant FTD patients.
7 Few studies have assessed insight in PPA, but evidence does point to loss of insight into select symptoms in this disorder. This is consistent with evidence from other neurological diseases, which demonstrate that loss of insight is sometimes quite radical, with a total denial of a particular symptom, but in other cases insight can be only partially diminished.
1,
9 The symptoms seen in dementias caused by FTLD can be grossly divided into two categories: behavioral symptoms such as disinhibition, apathy, or lack of spontaneity, and cognitive symptoms such as aphasia or difficulties with attention or executive functions. In the frontotemporal dementias and other disorders, including Alzheimer’s disease, studies suggest that behavioral symptoms are more vulnerable to loss of insight than certain cognitive symptoms.
10,
11 In PPA, language is the most affected domain. Few studies have assessed insight into language symptoms, although the evidence points to reduced insight in some patients with PPA,
12 with behavioral symptoms such as apathy most often associated with reduced insight.
10Levels of insight can be assessed by administering identical questionnaires to patients and their caregivers and calculating the discrepancy between their responses. This method has been applied to the study of insight into particular, isolated symptoms such as empathy
13 and disinhibition.
14 However, this technique has rarely been used to compare insight across a range of symptoms in a particular disease. The current study compared patient and caregiver ratings on a measure specifically designed to capture the spectrum of symptoms seen in FTLD, the Frontal Behavioral Inventory.
15 Questions on the Frontal Behavioral Inventory survey cognitive symptoms, including aphasia and inattention, and behavioral symptoms, including apathy and disinhibition. Assuming that the caregiver has more objectivity than the patient, this method permits the evaluation of patient insight into disease-related changes by comparing the total Frontal Behavioral Inventory scores in both groups. In addition, insight into specific symptoms can be investigated by comparing caregiver and patient scores on individual items.
The aims of this study were twofold. The first was to compare patient and caregiver scores on the Frontal Behavioral Inventory, with the hypothesis that preserved insight in the PPA group would be reflected in a smaller discrepancy between patient and caregiver scores than in the behavioral variant FTD group. The second aim was to assess symptom-specific insight by comparing the discrepancy between patient and caregiver scores on individual items on the Frontal Behavioral Inventory. For all groups, it was predicted that behavioral symptoms would be associated with greater discrepancy in symptom scores than cognitive symptoms.
RESULTS
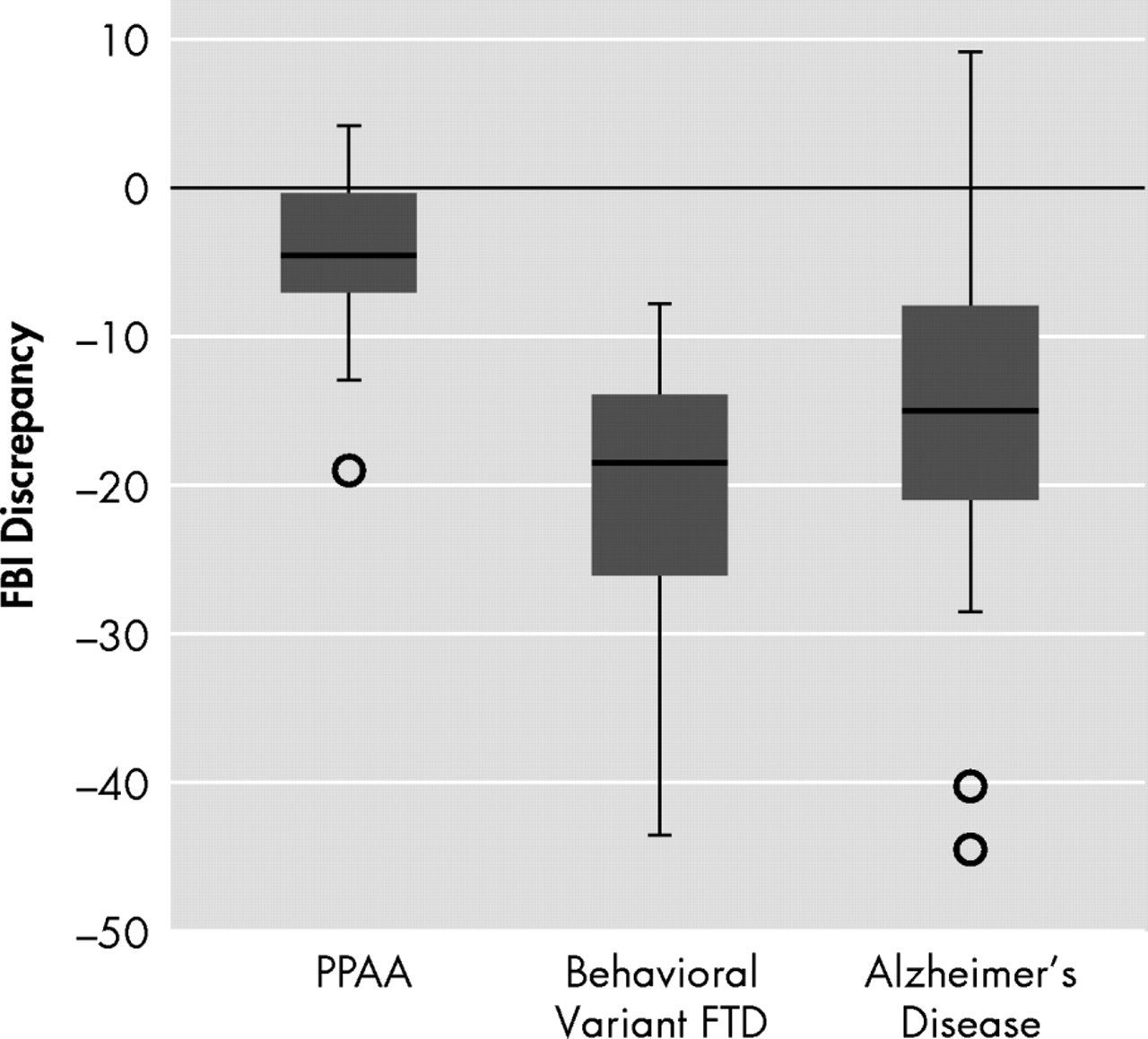
First, overall differences in insight among groups were compared using the Frontal Behavioral Inventory discrepancy score.
Figure 1 shows the distribution of scores. There was a significant effect of diagnosis (H=18.26, df=2, p<0.0005). Comparisons between groups indicated that the PPA patients’ discrepancy scores were significantly closer to zero, i.e., better patient-caregiver agreement than the behavioral variant FTD (U=9, p<0.0005) and the probable Alzheimer’s disease (U=61, p<0.0005) groups. The probable Alzheimer’s disease and behavioral variant FTD groups did not differ significantly. Data were also analyzed by dividing both the patient and caregiver total Frontal Behavioral Inventory scores by the number of symptoms that the caregiver endorsed, then comparing the difference between patient and caregiver scores within each of the groups. This step eliminates any bias resulting from the larger number of symptoms that behavioral variant FTD patients typically demonstrate on the Frontal Behavioral Inventory.
15 The same pattern found in the original analysis was evident (significant effect of diagnosis [H=19.15, df=2, p<0.0005]). There was no difference between the Alzheimer’s disease and behavioral variant FTD groups, and the PPA patients showed better agreement with their caregivers relative to the Alzheimer’s disease group (U=51.5, p<0.0005) and the behavioral variant FTD group (U=10.0, p<0.0005). In addition, given the wide variance in scores seen in each of the groups, correlations were performed with various disease-severity measures (MMSE, CDR, Activities of Daily Living Questionnaire
25 ) for each of the groups. None of these correlations were significant.
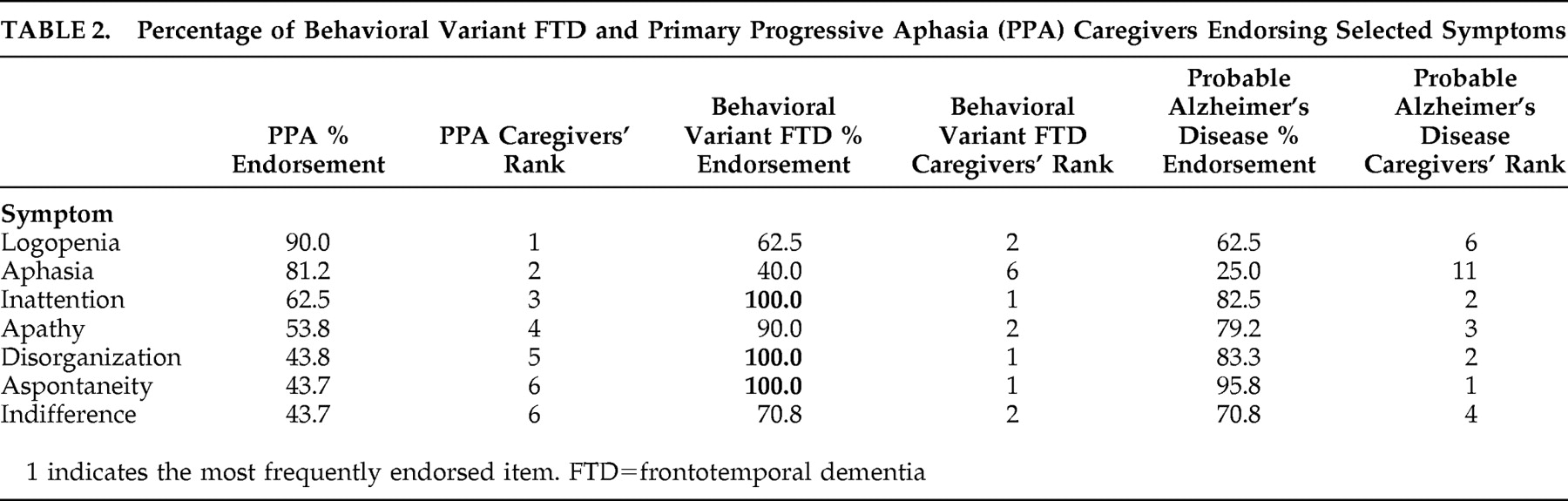
Subsequent to these analyses, symptoms were ranked in terms of the frequency with which they were endorsed by caregivers in order to select symptoms that would be used for symptom-specific analysis. Overall, symptoms were less frequently endorsed by PPA caregivers than by behavioral variant FTD caregivers. Therefore, the seven symptoms most frequently endorsed by PPA caregivers were selected. The frequency of endorsement and the ranking of these symptoms for the groups are presented in
Table 2 .
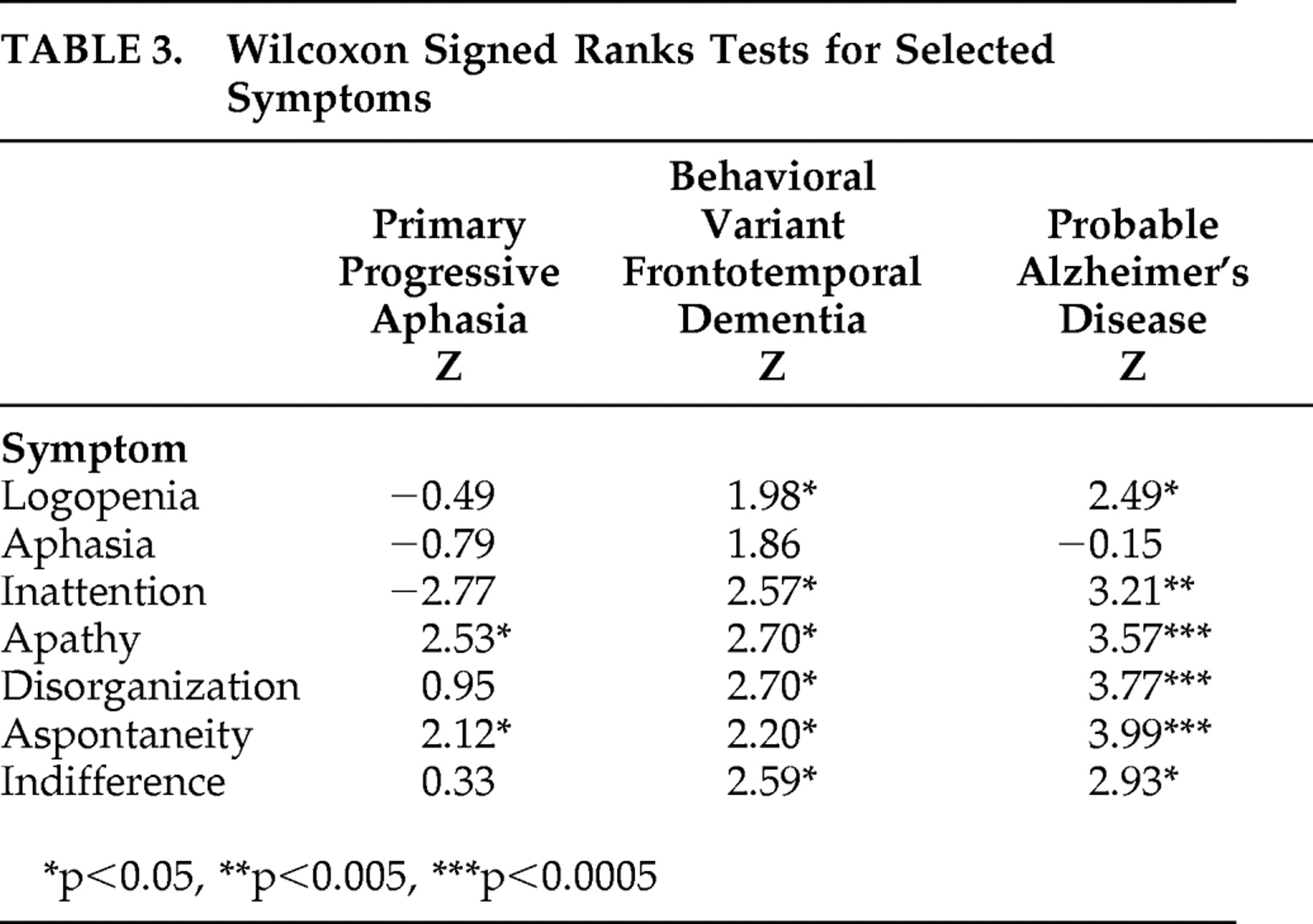
Results of the Wilcoxon signed ranks test on the Frontal Behavioral Inventory symptom discrepancy scores (patient score minus caregiver score) for each of the seven symptoms is displayed in
Table 3 . The scores indicated that the PPA group demonstrated significant differences between caregiver and patient responses only for apathy and aspontaneity, the behavioral variant FTD group differed significantly on all symptoms except aphasia, and the probable Alzheimer’s disease group differed significantly for all symptoms except aphasia.
DISCUSSION
Behavioral variant FTD patients were found to disagree with their caregivers more than PPA patients on their overall symptom frequency and intensity. However, there was substantial variability among patients in the behavioral variant FTD group, with some having similar levels of insight as PPA patients. Patients with PPA were more likely to be in closer agreement with their caregivers. There was also a relatively wide range of discrepancy scores within the PPA group, suggesting that some patients do, in fact, demonstrate poor insight. The comparison group of probable Alzheimer’s disease patients did not differ significantly from the behavioral variant FTD patients and showed the greatest variability in the degree of loss of insight. When symptom-specific insight was analyzed, behavioral variant FTD and probable Alzheimer’s disease patients disagreed with their caregivers for all symptoms analyzed except aphasia. Patients with PPA, however, disagreed with their caregivers only for apathy and aspontaneity. Patients with PPA tended to rate their language and other cognitive symptoms similarly to their caregivers’ ratings.
The majority of behavioral variant FTD patients in this study showed poor insight. However, there was a wide range of discrepancy on Frontal Behavioral Inventory scores in this group. The finding that some patients with behavioral variant FTD may have relatively intact insight was not expected, given that loss of insight is a core diagnostic criterion for behavioral variant FTD by Neary criteria.
4 This finding echoes that of a recent study investigating the empirical basis of this criterion.
5 Evers et al.
5 found three of eight behavioral variant FTD patients have intact insight when they used a semi-structured interview method directly asking patients about their disease. Another study found that even when behavioral variant FTD patients acknowledged their behavioral symptoms, they still did not express concern about how these symptoms might affect them or their families.
26 It may be that those behavioral variant FTD patients who were in closer agreement to their caregivers in the current study also demonstrated this pattern of anosodiaphoria (lack of concern regarding symptoms
27 ) as opposed to a frank anosognosia.
Another surprising finding was the lack of significant difference in levels of insight between the behavioral variant FTD and probable Alzheimer’s disease groups. The probable Alzheimer’s disease group also demonstrated the widest range of Frontal Behavioral Inventory discrepancy scores, suggesting that some of these patients had intact insight and others had poor insight. Previous studies regarding insight in probable Alzheimer’s disease also demonstrated variable levels of insight. The involvement of more frontal regions of the brain has been proposed as an explanation for reduced insight in some probable Alzheimer’s disease patients.
28 The lack of association between level of insight and disease severity measures further suggests that insight is not simply lost with progression of disease, but a more complex (likely neuroanatomical) explanation is needed.
The results of this study suggest that behavioral symptoms and non-language cognitive symptoms are more commonly associated with loss of insight than language symptoms. Research in various neurological disorders points to the symptom-specificity of insight.
29 –
31 With probable Alzheimer’s disease patients, Starkstein et al.
20 observed that insight for cognitive symptoms is distinct from insight for behavioral symptoms, and others have reported that loss of insight is commonly associated with behavioral symptoms in dementias caused by FTLD.
6 In our study, the behavioral variant FTD and probable Alzheimer’s disease patients behaved in a very similar manner in terms of symptom-specific insight, whereas PPA patients lost insight only into apathy and aspontaneity, which are behavioral symptoms, albeit closely related. Eslinger et al.
6 suggested that apathy and aspects of empathy were particularly sensitive to loss of insight in both behavioral variant FTD (they labeled these patients “Social/Dysexecutive” subtype) and the semantic dementia subtype of PPA; however, their behavioral variant FTD group differed from their PPA group in terms of insight into cognitive symptoms. The authors also found that behavioral variant FTD patients showed poor insight into cognitive symptoms such as memory and attention, whereas PPA patients complained of cognitive symptoms to a similar degree as their caregivers.
Numerous studies in probable Alzheimer’s disease have suggested that loss of insight into apathy is particularly common.
20,
32 It could be that apathy and insight are not, in fact, dissociable but that the lack of concern seen in apathy is conceptually related to anosognosia or, at least, anosodiaphoria. Attempts to treat apathy in neurological and psychiatric diseases with various medications have had mixed, but sometimes encouraging, results.
33 –
35 If insight and apathy are strongly intertwined, successfully reducing apathy with medication may affect insight. This concept has yet to be explored.
Some of the symptoms investigated (e.g., apathy, indifference) may also reflect mood disturbances, which have proved to be common in PPA
36 and Alzheimer’s disease
37 but not to the same degree in behavioral variant FTD.
38 These mood disturbances may relate to an increased rate of anosodiaphoria or may also disturb a mechanism of insight—somatic “tagging”
39 of information by the emotion network—making the information (in this case, about disease symptoms) more salient. Further research into this area is warranted.
Insight into aphasia was intact even in the behavioral variant FTD and probable Alzheimer’s disease groups whereas loss of insight for other cognitive symptoms was common. This is interesting in light of the underlying neuroanatomy. Aphasia is associated with damage to the left hemisphere perisylvian language region. Anosognosia, for the most part, is considered to be a right hemisphere phenomenon.
1,
40 Aphasia may in some way be “protected” from loss of insight due to the hemispheric localization of language. However, some patients with Wernicke’s aphasia appear to show loss of insight into their disordered speech despite the left hemisphere locus of damage.
41,
42 Those PPA patients who show reduced insight produce language with reduced meaningful content, similar to Wernicke’s patients.
12 However, it has yet to be established whether patients ever lose insight specifically into their language symptoms. The relationship between loss of insight in dementia and language symptoms warrants further investigation.
The lack of insight for even certain cognitive symptoms in behavioral variant FTD is consistent with findings in the literature that these patients lose their sense of “selfhood.”
43 It is possible that they no longer have a good sense of how they used to be, and hence they are unable to realistically compare their ability now to their ability before their disease onset. Rankin et al.
13 have revealed a tendency for behavioral variant FTD patients to overestimate positive aspects of their personalities such as assuredness and extroversion, while underestimating negative qualities such as cold-heartedness. It could be argued that behavioral variant FTD patients similarly hold a delusional belief that they are not impaired. During the disease process of behavioral variant FTD, patients may not passively lose awareness into their symptoms, but actively develop a new, positive self-view. Results from the current study suggest that PPA patients, in contrast, will sometimes actually be more critical of their abilities than their caregivers, potentially related to increased levels of depressive symptomatology in these patients.
36A number of important limitations to this study should be discussed. Our patients were matched in terms of disease duration, but differed on other indices of dementia severity. The Clinical Dementia Rating scale, with its emphasis on memory, may not be as accurate in assessing severity in non-Alzheimer’s dementias as it is in Alzheimer’s disease. Future studies may use other strategies in matching the patient groups. In addition, the patients were relatively homogenous in terms of disease duration, preventing analysis of the impact of this variable on insight. Discrepancy techniques, by definition, rely on the subjective responses of both patients and caregivers, which not only adds between-subject variability but may also be affected by factors other than the patients’ actual symptoms, such as caregiver distress (which has been previously found to be higher when the patient has reduced awareness
44 ) or quality of caregiver observation. This technique also fails to establish what aspect of insight is failing, for example whether there is a breakdown in general self-awareness or a more specific self-monitoring deficit (for discussions of these components of insight, see references
10,
29,
45 ). Finally, the group sizes, while typical of studies with these less common dementia populations, were not large. This limited the power of the statistical comparisons.
Reduced insight has important clinical implications in terms of caregiver burden, treatment compliance, and prognosis. Better understanding of this phenomenon in dementias caused by frontotemporal lobar degeneration will result in improved caregiver education, which has been shown to be beneficial in informing caregivers and enhancing coping strategies.
46 Currently, quantification of loss of insight is not a common part of the neurological or neuropsychological examination, but it may be an important addition, especially when loss of insight is considered a diagnostic
sine qua non for behavioral variant frontotemporal dementia.