Preoperative psychological and emotional factors have been shown to be related to pain and functional outcomes in orthopedic patients.
3 Also, Rosenberger et al.
4 suggested that preoperative consideration of attitudinal and mood factors will assist the surgeon in estimating both the speed and extent of postoperative recovery. Interaction between psychological factors and body functions in humans has been discussed by various investigators.
5 Apart from the hypothalamic-pituitary-autonomic nervous system, the cerebral cortex and limbic system should not be ignored.
6,
7 Although not in orthopedic patients, psychological factors have been related to the prognosis of cancer patients, and functional neuroimaging seems to be useful not only for psychiatric evaluation of major factors such as depression and anxiety but also for further psychological factors in cancer patients.
8 –
10METHODS
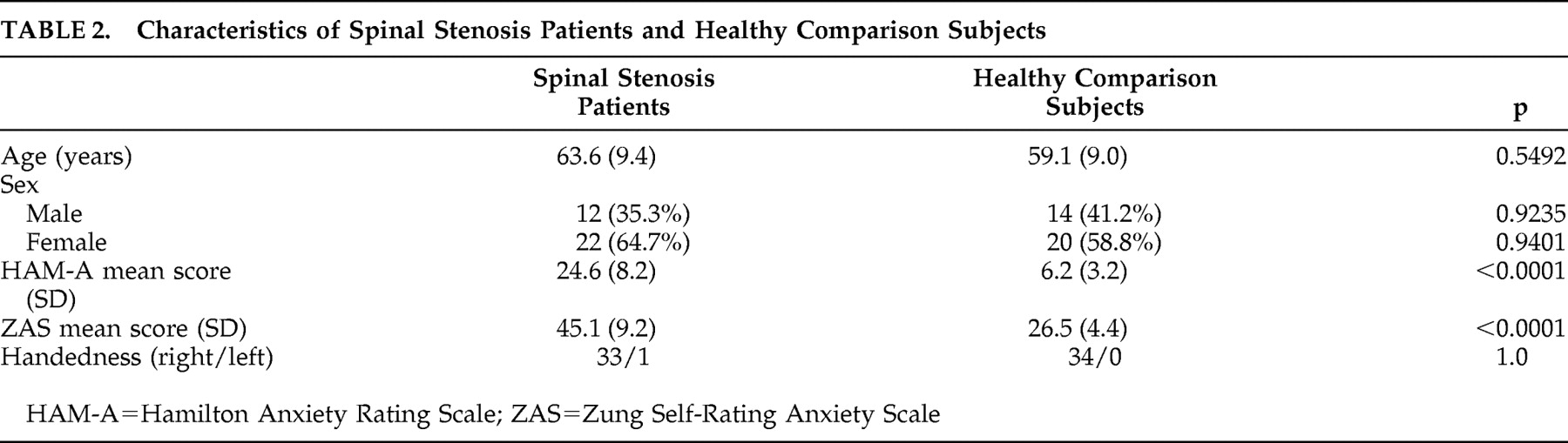
We studied 34 patients who had been admitted to our department for the surgical treatment of lumbar spinal stenosis. The diagnosis of lumbar spinal stenosis was based on clinical presentation and radiological findings (plain radiograph, computed tomography, and MRI). All patients were diagnosed as having lumbar spinal stenosis. All patients between 43 and 78 years old who presented spinal stenosis were asked to voluntarily complete a Hamilton Anxiety Rating Scale (HAM-A), Zung Self-Rating Anxiety Scale, and an analysis of their cerebral glucose metabolism.
17,
18The HAM-A consists of 14 items scored on a five-point scale. The Cronbach alpha for the HAM-A was 0.82. The Zung Self-Rating Anxiety Scale consists of 20 items scored on a four-point scale. The Cronbach alpha for the Self-Rating Anxiety Scale was 0.73. Thirty-four age- and gender-matched adults were used as the comparison group.
Before entering the study, all participants were also examined clinically to rule out any hidden metabolic disease or psychiatric disease that could affect the cerebral glucose metabolism. In addition, brain MRI studies were performed for all participants before entering the study in order to rule out organic brain lesions. Participants with a history of neuromuscular disease, endocrine disease, connective tissue abnormalities, organic brain disease, psychiatric disease, or previous spinal surgery were excluded from the study. All participants provided informed consent before the examination and measurements. The study was approved by the Clinical Research Ethics Committee of the university and the hospital.
FDG Brain PET
Brain positron emission tomography (PET) scans of a single frame of 15 minutes were acquired starting 60 minutes after the intravenous injection of 370 MBq (10 mCi) [ 18 F]fluorodeoxyglucose (FDG) using a Gemini PET/CT scanner (Philips, Milpitas, Calif.). Scans were performed while the participants were in a resting condition with their eyes closed and ears unplugged, comfortably lying in a darkened and quiet room. The participants fasted for at least 8 hours before PET imaging. The PET images were reconstructed using 3D RAMLA (2 repetition, 0.006 relaxation parameter) and displayed in a 128×128 matrix (pixel size=2×2 mm, with a slice thickness of 2 mm). Attenuation correction was performed with a uniform attenuation coefficient (μ=0.096 cm −1 ). In-plane and axial resolution of the scanner were 4.2 and 5.6 mm full width at half maximum (FWHM), respectively.
Statistical Parametric Map Analysis of Regional Cerebral Glucose Metabolism
Spatial preprocessing and statistical analysis were performed using the SPM2 implemented in Matlab 5.3 (The MathWorks, Inc., Natick, Mass.). All the reconstructed FDG brain PET images were spatially normalized into Montreal Neurological Institute (MNI, McGill University, Montreal, Quebec) standard templates by an affine transformation (12 parameters for rigid transformations) and a nonlinear transformation, and then smoothed with a FWHM 8 mm Gaussian kernel to increase the signal-to-noise ratio and to account for subtle variations in anatomic structures. To remove the effects of the difference in the overall counts, we normalized the voxel counts to the mean voxel count of the gray matter in each PET image using proportional scaling. Images of the spinal stenosis patients with preoperative anxiety were compared with those of healthy comparison subjects in a voxel-wise manner using SPM2 for between-group analysis (p<0.001, uncorrected; extent threshold, k=100). The clusters that passed this threshold were considered significant at p<0.05 corrected for multiple comparisons using family-wise error correction (Height threshold T=5.02, extent of cluster=100). The Talairach brain coordinates were estimated from a nonlinear transformation from MNI space to Talairach space (Talairach Daemon Client, Version 1.1, Research Imaging Center, University of Texas Health Science Center at San Antonio). We examined the differences between the spinal stenosis patients with preoperative anxiety and comparison subjects using an extent threshold level of 100 voxels with significance at p<0.001 to illustrate the group differences in statistical voxel-based analysis, as well as for illustrating the result of the registration between spinal stenosis patients with preoperative anxiety and comparison subjects. For the visualization of the t-score statistics (SPM{t} map), significant voxels were projected onto the 3-dimensional rendered brain or a standard high-resolution MRI template provided by SPM2, thereby allowing anatomic identification. In addition, we investigated linear correlations of the regional brain glucose metabolism with HAM-A and Zung Self-Rating Anxiety Scale scores using “single subject: covariates only” analysis and simple regression analysis, which are statistical models in SPM2 based on the general linear model.
Statistical Analysis
Statistical analysis was performed with SPSS 11.5 software for Windows (SPSS, Chicago). Data were expressed by mean and standard deviation (SD). We compared groups using the t test. Statistical significance was set at p<0.05.
DISCUSSION
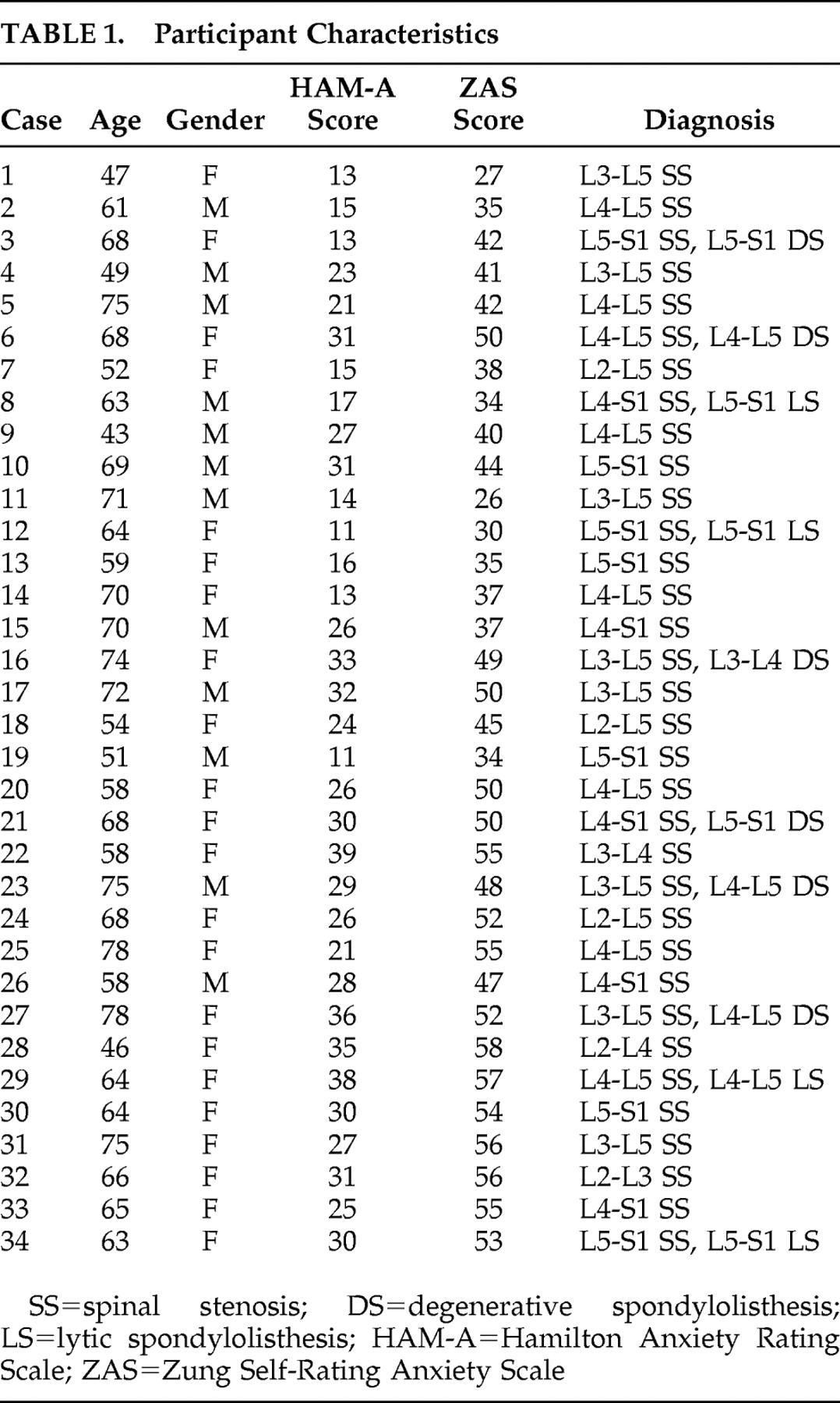
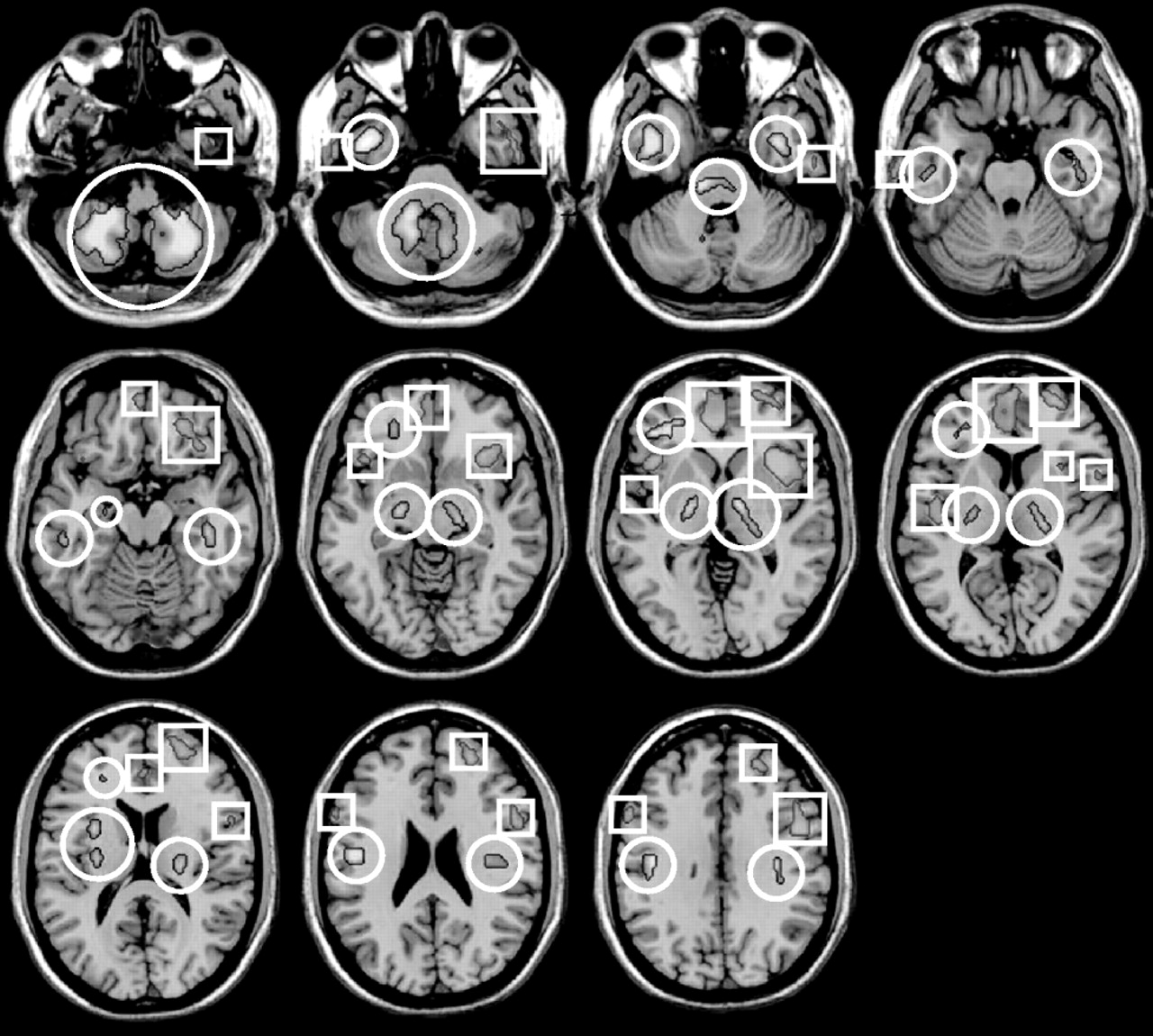
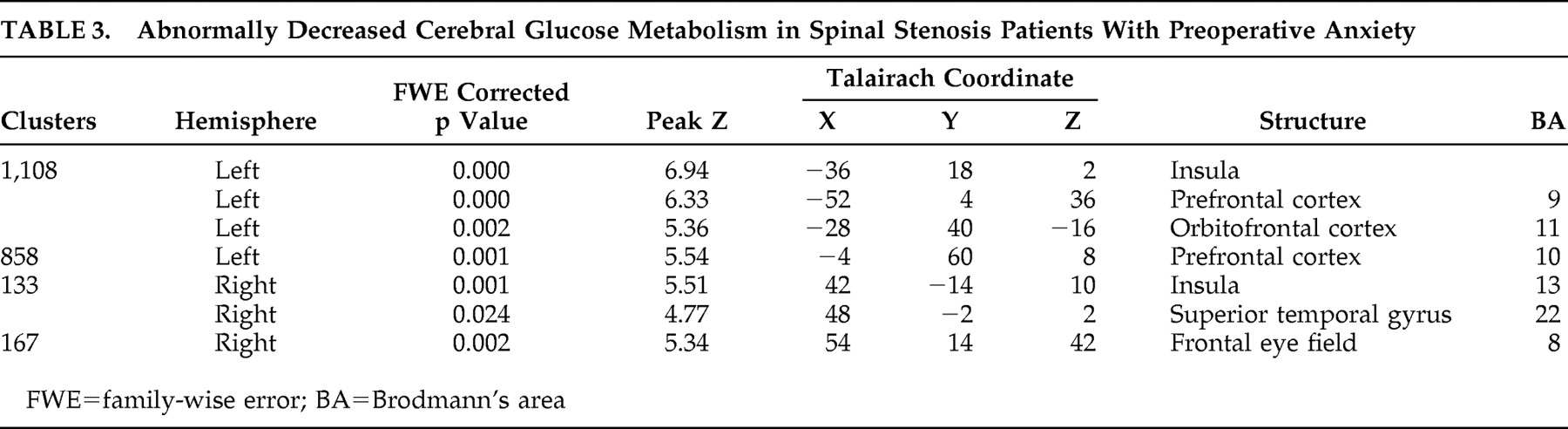
The major finding of our study is that spinal stenosis patients with preoperative anxiety showed decreased cerebral glucose metabolism in several areas, including the insula, temporal lobe, and frontal brain regions. Our study especially demonstrated left frontal brain hypometabolism.
Frontal brain asymmetry is suggested to be associated with differences in the basic dimensions of emotion.
19 The left prefrontal cortex is associated with the approach-related emotion, anger, whereas the right prefrontal area is associated with the withdrawal-related emotion, anxiety.
19,
20 Our study also showed hypometabolism of left prefrontal cortex and left orbitofrontal cortex in spinal stenosis patients with preoperative anxiety. Morinaga et al.
21 examined regional cerebral oxygenated hemoglobin (O
2 Hb) levels in human medial prefrontal cortex prior to and during anticipatory anxiety. They found that right medial prefrontal cortex O
2 Hb was significantly increased relative to left medial prefrontal cortex O
2 Hb during anticipation of the shock. Right-sided O
2 Hb increases were significantly correlated with the Temperament and Character Inventory Harm Avoidance subscale.
The orbitofrontal cortex and amygdala are supposed to be components of the neural circuitry involved in the adaptive processing of emotion and in psychopathology.
22,
23 A study of adolescent primates demonstrates involvement of the orbitofrontal cortex in mediating threat-induced freezing, a behavior that is similar to human behavioral inhibition and is a characteristic of trait-like anxiety.
24 They demonstrated that the orbitofrontal cortex lesions significantly increased left frontal asymmetric brain electrical activity, which is similar to findings in our study.
24Our study also revealed insular involvement in preoperative anxiety processing in spinal stenosis patients. Insular regions are known to be associated with modulation of affective processing and are important for linking emotions to cognitive processes and behavioral responses.
25 Several studies have noted that the insular cortex may be central for understanding anxiety proneness. In particular, the insular cortex is supposed to process visceral responses accessible to awareness as subjective feeling states.
26 Some studies have found the altered insular function in anxiety disorders. The insular is activated during anticipatory anxiety paradigms in healthy subjects and in patients with social anxiety disorders.
27 –
29In our study, decreased cerebral glucose metabolism was noted in the right superior temporal gyrus (Brodmann’s area 22) of preoperative anxiety patients with spinal stenosis. This finding is consistent with prior studies which found structural abnormalities of the temporal lobe in panic disorder patients.
30,
31 Other studies also have reported the structural abnormalities of the temporal lobe in patients with panic disorder. The right temporal lobe is believed to be associated with an earlier onset and frequent panic attacks in patients with panic disorder.
32 Similar to the current study, a PET study noted the decreased cerebral glucose metabolism in the right inferior parietal and right superior temporal regions.
33 The authors assumed that the temporal lobe played a mediating role between affect and behavioral responses with input from the limbic system. The authors also suggested that decreased metabolism in the right superior temporal region would be associated with an elevated γ-amino-butyric acid (GABA) activity receptor system in the right prefrontal cortex. In addition to metabolic changes, hemodynamic alteration in panic disorder patients is also reported. A recent study demonstrated that there is decreased cerebral blood flow in temporal regions of the brain in panic disorder and that this decrease may, in part, reflect the clinical severity of panic disorder.
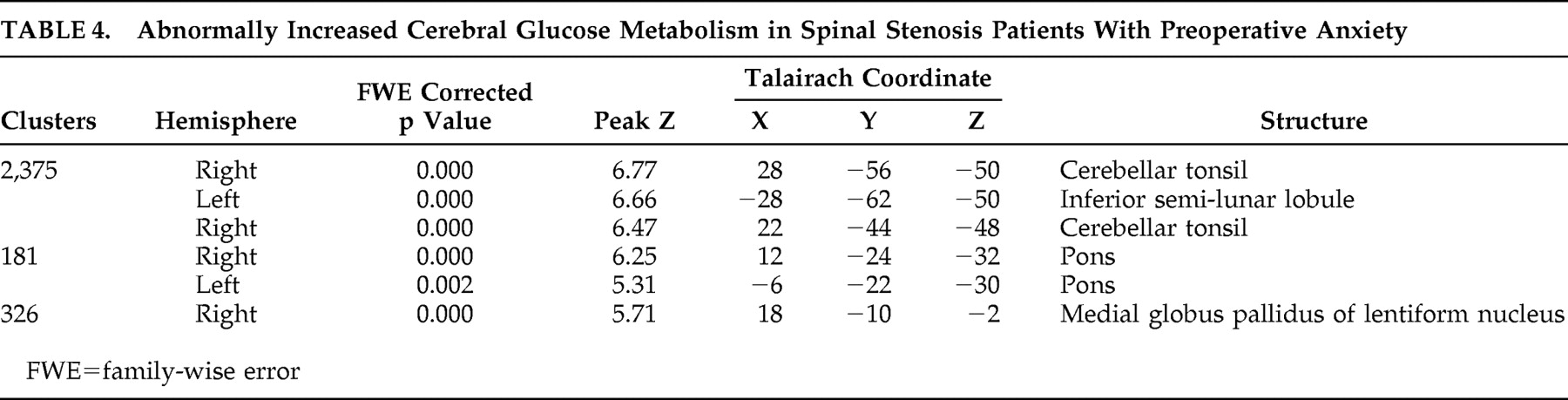
34Interestingly, spinal stenosis patients with preoperative anxiety showed increased cerebral glucose metabolism in several brain areas, including right cerebellar tonsil, left inferior semilunar lobule of cerebellum, right cerebellar tonsil, both pons, and right internal globus pallidus. These findings suggest the hyperactivity of two neurocircuits: afferent pathways to the amygdala and part of the startle circuit. The afferent pathways of the fear network include the viscerosensory information conveyed by the nucleus of the solitary tract, the visuospatial/auditory or cognitive information from the thalamus, and fear-signifying memories from the hippocampus.
35 Previous preclinical studies have found an efferent amygdalofugal pathway to the primary startle circuit at the level of the caudal pontine reticular formation, which is relayed by the periaqueductal gray matter. Higher FDG uptake may have been detected in the lower dorsal pons and midbrain, associated with the anticipatory anxiety of panic disorder patients.
36Sacchetti et al.
37 reported on the relationship between cerebellar regions and panic disorder, which is a role of the cerebellum in fear-conditioning consolidation. The study showed that interpositus nucleus functional integrity was necessary for acoustic, conditioned stimulus fear response memory formation and that vermis functional integrity was necessary for memory formation of both context and acoustic, conditioned stimulus fear responses. Also, similar results were found in the study by Sakai et al.
38 in which panic disorder patients showed appreciably high-state anxiety before scanning, and exhibited significantly higher levels of glucose uptake in the bilateral amygdala, hippocampus, and thalamus, and in the midbrain, caudal pons, medulla, and cerebellum than comparison subjects.
Interestingly, there are recent reports of panic with autonomic disturbances occurring in patients receiving hypothalamic deep brain stimulation for cluster headaches and for deep brain stimulation in the subthalamic nucleus region.
39,
40 Data indicate that subthalamic nucleus deep brain stimulation can both impair fear recognition and induce fear and panic.
39,
41 A similar finding was reported in 2006.
42 The authors of that study showed that deep brain stimulation of the anterior limb of the internal capsule and nucleus accumbens region caused severe panic, and this response may result from the activation of limbic and autonomic networks.
A major drawback of our study is that the difference in the severity of the anxiety between the healthy comparison group and the spinal stenosis patients with preoperative anxiety is a potential confound. In other words, the lack of participant anxiety scores is the major limitation of the current study. It is possible that stability in anxiety in pre- and postoperative participants might be related to stability in trait anxiety in this group. In other words, perhaps people who are by nature highly anxious are more likely to undergo surgical procedures or are at greater risk for health-related problems. Therefore, this major problem should be addressed in a future study.
Spinal stenosis patients with preoperative anxiety showed decreased cerebral glucose metabolism in several brain areas including the left insula, left prefrontal cortex, right insula, right superior temporal gyrus, and right middle frontal gyrus. Increases were also noted in the right cerebellar tonsil, left inferior semilunar lobule, right cerebellar tonsil, both pons, and right internal globus pallidus. The findings of our study provide functional neuroimaging support of abnormal cerebral glucose metabolism in spinal stenosis patients with preoperative anxiety.