N europsychiatric symptoms are common in both people with dementia and people who have sustained a traumatic brain injury (TBI).
1 Common neuropsychiatric symptoms in dementia and post-TBI include depression, apathy, irritability, agitation, aggression, wandering, change in sleep pattern, delusions, and hallucinations.
2 –
4 These symptoms can present as a psychiatric syndrome, such as major depression, or can occur in isolation. In dementia, neuropsychiatric symptoms are a major source of suffering to both patients and caregivers by interfering with sleep,
5 impairing quality of life,
6 leading to institutional placement,
7 and compounding caregiver financial burden and emotional stress.
8 While patients affected by dementia and TBI independently are at increased risk for neuropsychiatric symptoms, no published data have examined the question of whether people with dementia who have sustained a TBI in the past are at increased risk for developing neuropsychiatric symptoms. Given that dementia and TBI are both associated with damage to the frontal cortex and associated frontal-subcortical circuits, and damage to these circuits from a variety of insults predisposes patients to psychopathology,
9 we hypothesized that, in persons with dementia, TBI prior to the onset of dementia would be a risk factor for increased neuropsychiatric symptoms associated with frontal lobe damage such as disinhibition, irritability, depression, apathy, and aggression. We examined this question in a population-based study of persons with dementia whose history of TBI was assessed prior to the onset of dementia and in whom dementia-onset was assessed prospectively (incident cohort).
METHODS
This is a prospective study involving persons with incident dementia who took part in the Cache County Study on Memory, Health, and Aging (CCSMHA). The CCSMHA is an ongoing population-based study of the epidemiology and progression of dementia. The study has been approved by Institutional Review Boards at Duke University Medical Center, Johns Hopkins University, and Utah State University. Informed consent was obtained from either the study participants or appropriate next of kin. This study has been described previously.
10,
11 Briefly, a total of 5,092 respondents (5,677 were invited) ages 65 years and older participated in the study. All participants underwent a multistage screening and assessment for prevalent dementia (Wave 1)
. Those with dementia in Wave 1 and those that did not develop dementia or dropped out between Waves 1 and 3 were excluded from these analyses (n=4,265).
TBI Assessment
Head injury was assessed prior to the onset of dementia, mainly from retrospective reports provided by the participants, and updated by collateral informants at the time of dementia diagnosis. In addition, all participants and informants were asked if they had ever lost consciousness following a head injury. For the purpose of these analyses, those with a history of head injury and loss of consciousness were considered to have had a TBI, since loss of consciousness is a good indicator of at least moderate brain injury.
Dementia Assessment
The study originally enrolled 5,092 individuals who were all screened using a revised version of the Modified Mini-Mental State Examination (3MS),
12,
13 or with the Informant Questionnaire for Cognitive Decline in Elderly (IQCODE)
14 for those unable to complete the 3MS. Participants who had an education and sensory adjusted 3MS score <87, who had an IQCODE score ≥3.27, or who were 90 years old or older were further assessed using the Dementia Questionnaire.
15 Participants with suspected dementia underwent a comprehensive clinical assessment for dementia in their homes. Additionally, participants constituting a 19% weighted age-, gender- and genotype-stratified probability subsample selected regardless of performance at the two screening stages were invited to complete the clinical assessment. The clinical team included a research nurse and a psychometric technician. Those with a provisional diagnosis of dementia were invited to complete an examination by a geriatric psychiatrist and to undergo MRI and laboratory studies. At a consensus conference, data from these evaluations were used to diagnose participants with dementia using DSM-III-R criteria. Diagnoses of Alzheimer’s disease followed the criteria from the National Institute of Neurological and Communicative Disorders and Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA),
16 and vascular dementia, the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN).
17 The team of experts who diagnosed participants at the consensus conference included two geriatric psychiatrists, a board-certified neurologist, two neuropsychologists, and a cognitive neuroscientist.
10,
18 Similar procedures were followed at each incident wave, with the exception of slight modifications to the 3MS screening score and elimination of the Dementia Questionnaire at Wave 3. Additionally, at Wave 3, all persons completing the screening visit who were aged 85 and older were selected for the clinical assessment.
Assessment for Neuropsychiatric Symptoms
Neuropsychiatric symptoms were assessed at the clinical assessment using the Neuropsychiatric Inventory (NPI),
19 a widely used instrument in dementia research. It is an informant-based interview that is well validated with excellent interrater reliability. The NPI was administered to caregivers or to persons very familiar with the participants. The NPI covers 10 domains: delusions, hallucinations, agitation/aggression, dysphoria/depression, anxiety, euphoria/elation, apathy/indifference, disinhibition, irritability/lability, and aberrant motor behaviors. Each domain has a screening question, with a positive answer being followed up by a detailed set of questions about the domain. Symptom severity was assessed by then asking the informant to rate both the frequency and severity of symptoms which produces a composite domain-specific frequency × severity score.
20 Since no participants endorsed the euphoria/elation domain, it was excluded from the analyses. Total NPI score was calculated by summing the composite domain specific severity ratings for the remaining nine domains. Higher scores indicate greater overall neuropsychiatric symptom severity.
Covariates
Age, gender, and apolipoprotein E (
APOE ) genotype obtained using DNA samples were included in the analyses as covariates because these are risk factors for neuropsychiatric symptoms.
21 –
23 Those who had one or two
APOE 4 alleles were grouped together as
APOE 4 positive and those without any
APOE 4 allele as
APOE 4 negative.
APOE genotypes were determined following the method of Richards and colleagues
24 using polymerase chain reaction amplification and a restriction isotyping method described by Saunders and colleagues.
25Statistical Analysis
We used t tests for continuous variables and chi-square tests for discrete variables to examine demographic differences between groups with and without TBI. The two groups were also compared on the severity of NPI symptoms using t tests. Two sets of binary logistic regression models were generated to determine the odds of having, as opposed to not having, specific neuropsychiatric symptoms based on the presence or absence of TBI. The first set of models estimated the odds of endorsing specific neuropsychiatric symptoms based on only the presence of TBI. The second set estimated an adjusted odds ratio, based on the presence of TBI, adjusted for APOE -4, gender, and age.
RESULTS
Of the 453 participants with incident dementia at either Wave 2 or Wave 3, 125 (27.6%) had a lifetime history of head injury. Of those, 79 reported head injury with loss of consciousness, 42 reported head injury but no loss of consciousness, and four reported head injury but unclear history of loss of consciousness. Using the definition of TBI described previously, we compared those with history of head injury and concomitant loss of consciousness (n=79) to those without (n=370), thus considering those with TBI but no loss of consciousness as part of the comparison group. The four people with unclear history of loss of consciousness were excluded from the analysis.
Dementia Diagnosis
Of the total 449 included in the analysis, 271 (60.4%) were diagnosed with Alzheimer’s disease, 31 (6.9%) with Alzheimer’s disease and vascular dementia, 16 (3.6%) with Alzheimer’s and other dementia, 50 (11.1%) with vascular dementia, and 81 (18%) with other dementias.
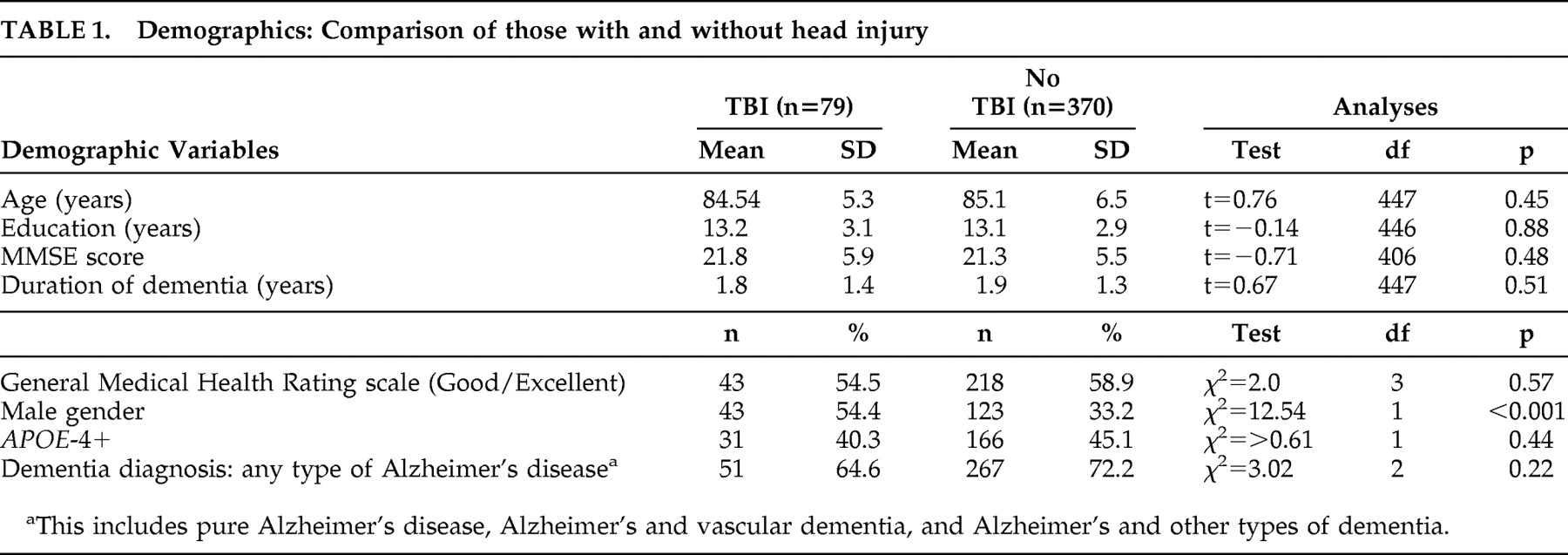
Comparison on Demographic Variables
When comparing those with TBI (n=79) to those without (n=370), the TBI group was more likely to be male (54% versus 33%; χ
2 =12.5, p<0.001). No significant group differences were found on any of the other demographic or clinical variables (
Table 1 ).
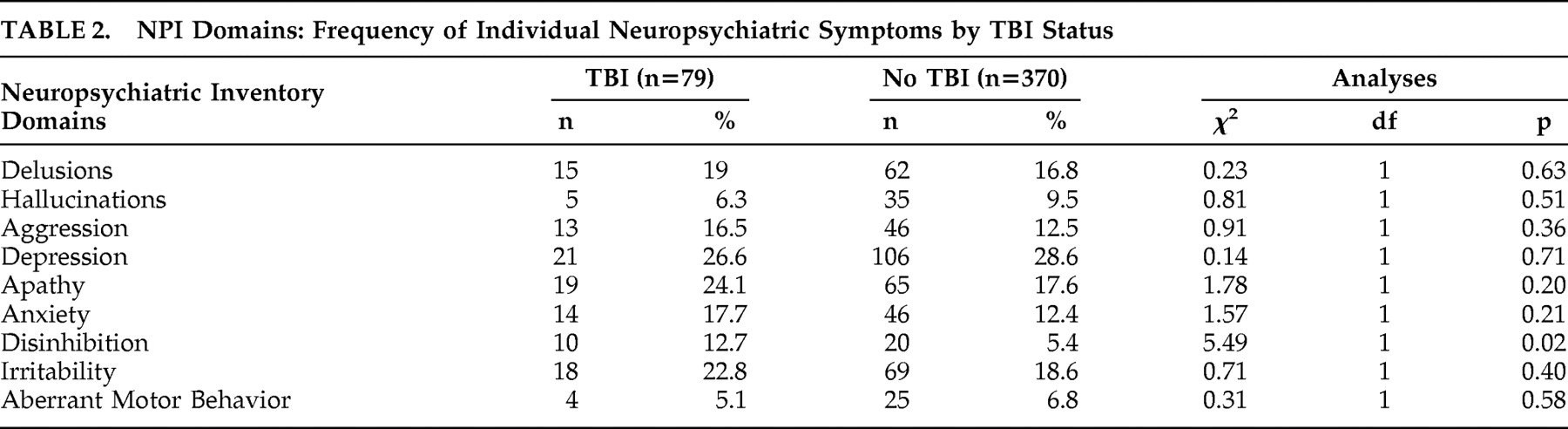
Comparison on Neuropsychiatric Symptoms
A greater percentage of participants with TBI than without TBI endorsed exhibited disinhibition (12.7% versus 5.4%; χ
2 =5.5, p=0.02) (
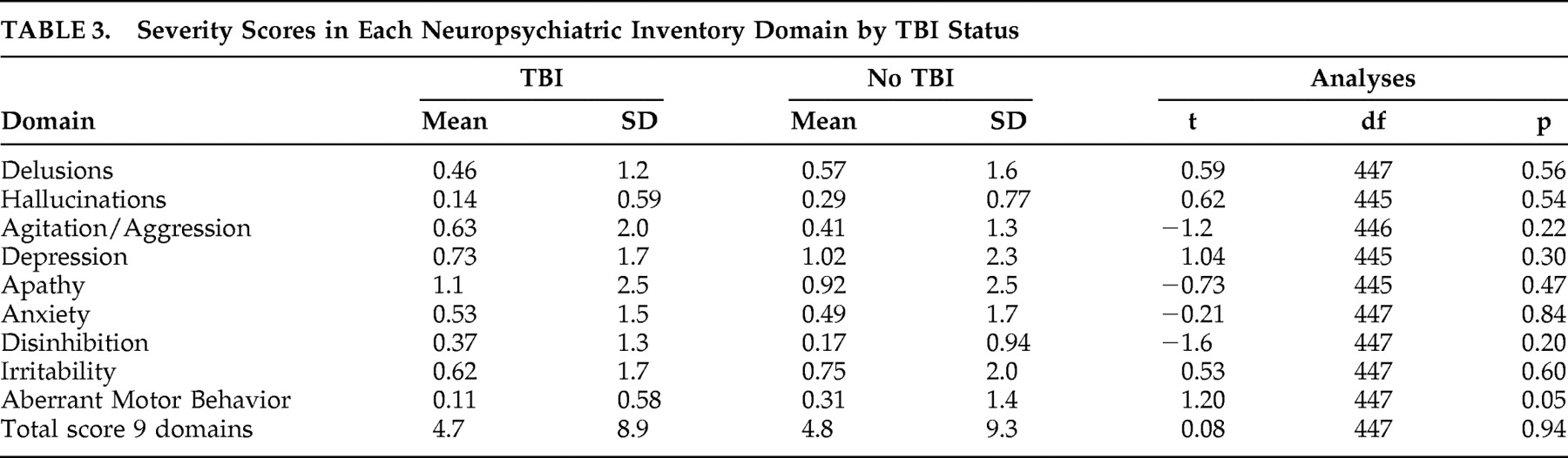
Table 2 ). There was no significant difference between those with and without TBI on any of the other NPI domains. Similarly there were no significant differences between those with and without TBI on the severity scores of the NPI domains (
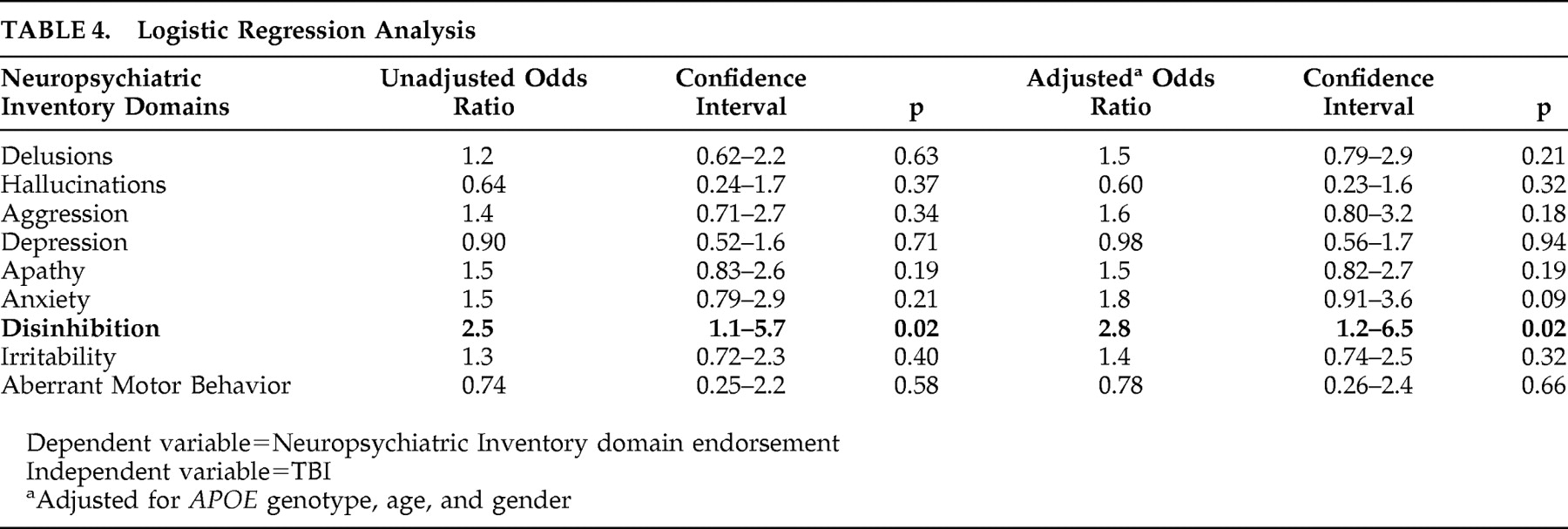
Table 3 ) or on the total NPI score. Binary logistic regression models were estimated for each NPI domain to estimate odds ratios of having individual neuropsychiatric symptoms by TBI status. A lifetime history of TBI increased the odds of having disinhibition nearly threefold (OR=2.8, p=0.02) but was not associated with elevated risk of other neuropsychiatric symptoms (
Table 4 ).
Exploratory analyses were carried out to determine if the time since the most recent TBI affected the occurrence of neuropsychiatric symptoms. Of the 79 with TBI, 68 were able to provide information on the date of the most recent TBI. The duration from the most recent TBI to dementia diagnosis ranged from less than 1 year to a maximum of 85 years, with a mean of 42.8 years and a median of 47 years. The TBI group was divided into 2 subgroups, using a mode split: those with 14 years or more since TBI and those with 13 years or less since TBI. Multiple modes existed, but 14 years was chosen as this was the lowest value. No significant differences were noted on the occurrence of neuropsychiatric symptoms between the two groups.
DISCUSSION
In a population-based cohort of persons with incident dementia identified, the report of a lifetime history of TBI with a loss of consciousness prior to the onset of dementia was associated with a higher prevalence of disinhibition, but not with other types of neuropsychiatric symptoms. Those with a history of TBI had almost threefold increased odds of developing disinhibition compared to those without a history of TBI (p=0.02). Adjustment for
APOE 4 genotype, gender, and age did not diminish this relationship. This is of potentially great clinical significance since disinhibition in dementia is associated with serious consequences for patients and their caregivers.
26 The occurrence of any type of neuropsychiatric symptoms in people with dementia is associated with adverse consequences including worse quality of life, greater disability, accelerated cognitive or functional decline, greater burden on caregivers, earlier institutionalization, and increased mortality.
27 Behavioral problems are more burdensome to caregivers than cognitive or physical problems.
7,
28 There are few prior reports of disinhibition alone as a neuropsychiatric symptom in dementia, and the data presented here suggest that dementia patients with TBI may merit special attention particularly to this symptom. There have not been trials of interventions specifically for disinhibition, and it is not clear that efforts at treating agitation/aggression will generalize to disinhibition.
29,
30 New treatment approaches may be needed.
Currently, there is no approved drug for the treatment of disinhibition in dementia. Behavioral treatment is the first line of management of this complicated problem. There are case reports of successful treatment of sexual disinhibition with gabapentin
31 and carbamazepine.
32 Kraus and Maki
33 have reported a positive response in seven brain-injured patients with frontal lobe dysfunction.
Our finding underscores the importance of counseling caregivers regarding the risk of disinhibited behavior in dementia patients with history of TBI. Regular follow-up visits and maintaining communication with the caregivers often help in early detection of this symptom. As disinhibition often responds poorly to psychotropics, management should focus on maintenance of structure and routine, environmental limit setting, supportive therapy and respite for caregivers.
While we confirmed our hypothesis of an association between TBI and disinhibition, we failed to confirm an association between TBI and other neuropsychiatric symptoms. We had hypothesized that those with TBI would have a rate of other neuropsychiatric symptoms associated with injury to frontal-subcortical loops, such as depression, irritability and apathy, compared to those without. Possible explanations are: (a) as all participants were newly diagnosed dementia patients, most of them may be in the early stages of dementia and behavioral problems may not yet have developed; (b) there may be a critical period in the later stages of dementia when a synergistic effect of TBI begins and behavioral problems are exacerbated; (c) TBI may increase the risk of experiencing certain behavioral symptoms such as disinhibition, but the severity of these symptoms may not be different from those without TBI; (d) TBI may not be a risk factor for increased behavioral symptoms in dementia.
Our inability to detect other behavior problems in those with and without TBI could also be due to inadequate assessment of TBI. Traumatic brain injury was assessed by a single question about history of loss of consciousness, which inevitably misses many potential fine points of diagnosis such as the severity of injury and the distinction between closed- and open-skull injuries. In addition, it relies on retrospective assessment and inevitably involves recall bias. It is not predictable whether these confounds lead to over- or underestimation of TBI. The most appropriate method to diagnose TBI is by obtaining a careful history on the cause, nature and severity of TBI from both the person and collateral informants, reviewing old medical records, and reviewing brain films done at the time of injury and thereafter. Nevertheless, our assessment and definition of TBI are in line with previously published studies and are considered a viable method when records are not available and direct exam is not possible.
Despite these limitations, this is the first study to assess the role of TBI in precipitating neuropsychiatric symptoms in early dementia. At this point in the course of dementia, relatively few problems are expected. The increased odds of displaying disinhibition early on in dementia patients with history of TBI is concerning as it can interfere with treatment and lead to early institutionalization. Further, the study is strengthened by the homogeneity of the study population. They are homogenous in several aspects including low smoking and drinking rates, high level of education, longevity, and low out-migration rates. These characteristics make this population a unique sample but also help to control for factors that could confound the results.
CONCLUSION
Several research questions arise from this finding, which can be better answered in longitudinal studies. What is the difference in progression of dementia illness between those with and without TBI? Do they have differing rates and severity of behavioral symptoms as dementia progresses? Are there specific domains such as disinhibition that have a different longitudinal trajectory in later stages of dementia for those with TBI compared to those without? Continued longitudinal follow-up on this sample will help answer these questions.
Acknowledgments
This work was supported by NIH grants AG11380, AG18712, and AG21136. We thank the neurogenetics laboratory of the Bryan Alzheimer’s Disease Research Center at Duke University for the APOE genotyping. We are grateful to Drs. Kathleen A. Welsh-Bohmer and John C. S. Breitner, current and former principal investigators of the Cache County Study, for sharing the data and for their support and encouragement of this manuscript.
These results were presented as a poster by DP at Northeastern Ohio Universities College of Medicine’s Summer Research Fellowship Program on 9/3/08 in Rootstown, Ohio, and as an oral presentation at Summa Health System on 1/8/09 in Akron, Ohio. DP is a Medical Student Training in Aging Research (MSTAR) scholar and presented these results at the 2009 American Geriatric Society Annual Scientific Meeting.