Oliveri et al.
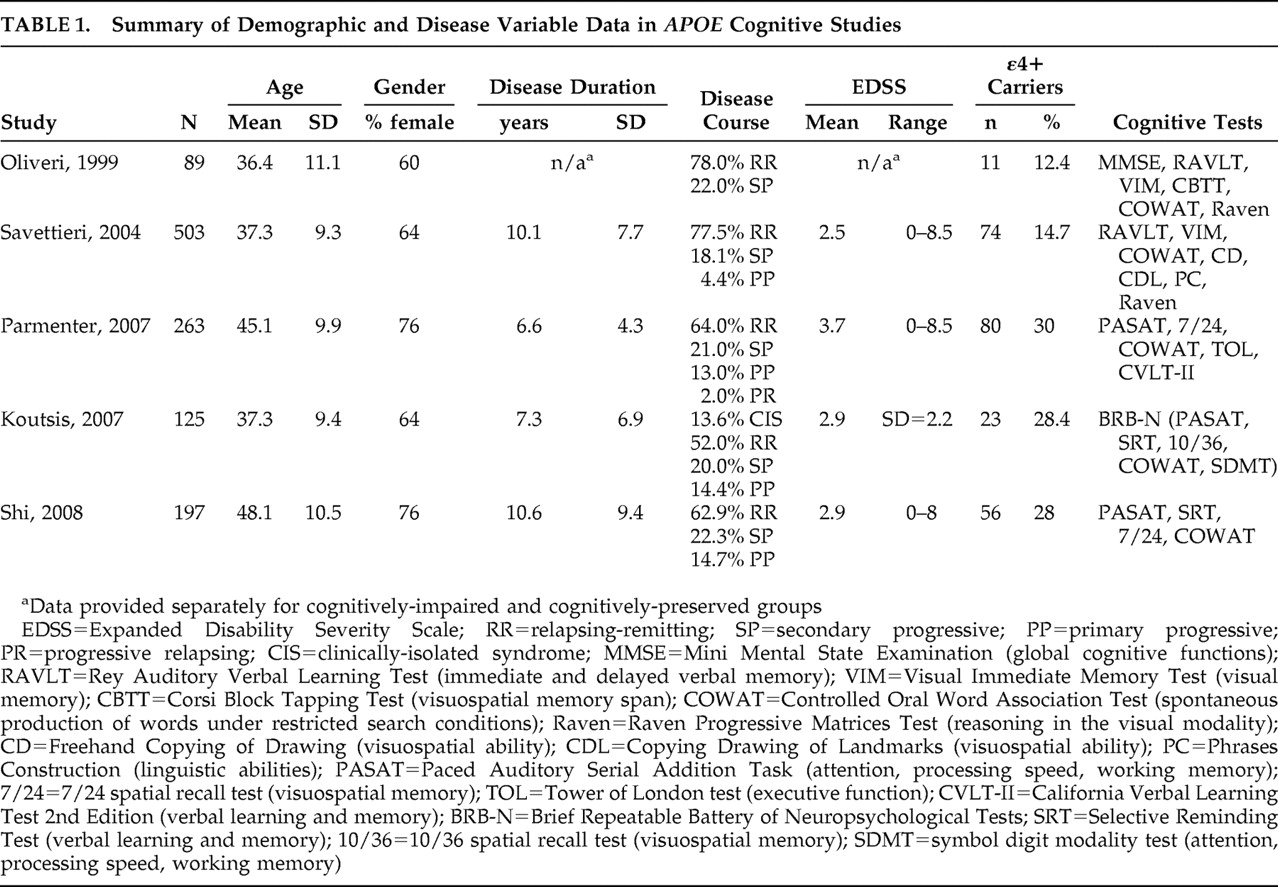
54 studied 89 multiple sclerosis subjects, 12 of whom were ε4+. Demographic and disease variables were provided for the entire sample as shown in
Table 1, but how these factors compared between ε4+ and ε4− groups was not specified. Based on a brief battery of tests, some of which are seldom used in multiple sclerosis research, the neuropsychological scores of multiple sclerosis patients were compared to age-, gender-, and education-matched healthy control subjects in previous studies. Patients were classified as cognitively impaired if their results on at least one cognitive test were deemed to be abnormal. The criteria for this designation were again unspecified. No difference was found in the frequency of the three alleles (ε2, ε3, ε4) and four genotypes (ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4) between cognitively impaired and cognitively preserved multiple sclerosis subjects. Acknowledging the small size of the ε4+ group, the authors concluded that their data did not support an adverse effect of the ε4 allele on cognition in multiple sclerosis. A subsequent revision of these analyses produced a different result, however.
55 With multiple sclerosis subjects divided into one of two groups based on ε4 carrier status, a significantly higher proportion of ε4+ patients were present in the impaired group.
Savettieri et al.
56 studied 503 multiple sclerosis patients also using a brief cognitive battery (
Table 1 ). As in the previous study, no data were provided on whether ε4+ and ε4− subjects were matched on demographic and disease variables. Cutoff scores for cognitive impairment were derived from a previous study of normative data. Failure on one to four tests signified mild impairment, while those failing five or more tests were characterized as having severe impairment. In the primary analysis, ε4+ subjects were represented in equal proportions in the cognitively impaired (mild + severe) and preserved groups. However, the cognitively impaired group had significantly more males, longer disease duration, more secondary progressive disease, lower education, and more physical disability. Taking these potential influences into account, the authors performed a number of subgroup analyses that ultimately led them to conclude that high EDSS, low education, and ε4 carriage were significant risk factors for severe cognitive impairment in males. In females, no variables were identified as risk factors for cognitive impairment.
In the third study,
55 ε4 carriers were matched to ε4 noncarriers on age, gender, education, duration of multiple sclerosis, age at onset of multiple sclerosis, EDSS, depression, anxiety, and fatigue (
Table 1 ). The often-cited, validated Neuropsychological Screening Battery for multiple sclerosis (NSBMS)
1 was modified by substituting the Selective Reminding Test with the California Verbal Learning Test—II (CVLT-II). Patients’ raw scores on each cognitive test were converted to z scores based on published means from normative samples. The z scores from all tests were then averaged and transformed to a single standard cognitive composite score, which did not differ between ε4+ and ε4− groups. Two categorical analyses were then undertaken. The first dichotomized the sample into cognitively intact and cognitively impaired, with the latter defined as having a standard cognitive composite score at least one standard deviation below the mean (n=95, 40% of the sample). No significant differences were found with respect to the frequency of ε4 carriage in these groups. In the second analysis, a stricter criterion for cognitive impairment was used. To be classified as impaired, a subject had to score at least one standard deviation below the mean on the Paced Auditory Serial Addition Task (PASAT), on two or more measures derived from the CVLT-II, and on two or more of the remaining tests in their battery. This substantively reduced the size of the cognitively impaired sample (n=25, 11% of the sample). However, with the stricter criterion, 23% (13/56) of ε4 carriers were deemed to be cognitively impaired, compared to only 8% (12/150) of non-ε4 carriers, a statistically significant difference.
In the fourth study, Koutsis et al.
59 used the Brief Repeatable Battery of Neuropsychological Tests (BRB-N), which consists of the four tests in the NSBMS plus the Symbol Digit Modalities Test
60 (
Table 1 ). The cutoff for failure on an individual cognitive test was designated as below the fifth percentile of scores obtained by age, gender, and education matched controls (n=43), a threshold deemed by previous studies to be clinically meaningful.
1,
2 Overall cognitive impairment was defined as failure on at least three tests. ε4 carriers did not differ from non-ε4 carriers on age, gender, education, age at onset, disease duration, disease course, EDSS, disease-modifying drugs, or depression. Potential differences in the frequencies of overall and domain-specific cognitive impairment were analyzed by logistic regression with ε4 status as a predictor variable. This showed that presence of the ε4 allele did not affect the risk of overall cognitive impairment. However, of the individual cognitive domains defined, ε4 carriage did emerge as a significant predictor of verbal learning impairment with a sixfold increase in relative risk of failure compared to noncarriers. Failure of verbal learning was defined as failure on both long-term storage and consistent long-term retrieval components of the Selective Reminding Test (SRT). The key finding of this study was the link between ε4 and a specific cognitive domain, namely verbal memory, a finding obscured by composite cognitive score.
The possibility of domain-specific cognitive impairment in ε4+ multiple sclerosis patients was also addressed in the next study. Shi et al.
61 used the NSBMS to study ε4+ subjects who were slightly older (mean=50.9, SD=9.3 years) than ε4− subjects (mean=47, SD=10.8 years, p=0.017). The two groups were otherwise similar with respect to other demographic and disease parameters. Raw scores for each cognitive test were converted to standardized t scores corrected for age, gender, and education, and the cutoff for failure was taken as below the fifth percentile of the t score. The odds ratio of failing one index of the verbal memory test (Selective Reminding Test), namely, consistent long-term retrieval, in ε4+ versus ε4− patients was 2.1 (p=0.035). There were no other significant cognitive differences. In a secondary analysis, the authors compared a subgroup of the youngest ε4 carriers to age-matched noncarriers (mean=36.4, SD=2.4 years, range=31 to 40 years) and found that a significantly higher proportion of ε4 carriers had verbal memory impairment. When the overall sample was stratified by age (decades), the young cohort of ε4+ patients (ages 31–40) was found to be especially susceptible to verbal learning deficits. These secondary results are difficult to interpret, however, since subgroup sample sizes were not provided.
Magnetic Resonance Studies of APOE in Multiple Sclerosis
Brain pathology as elucidated by MRI is a robust predictor of cognitive impairment. Significant correlations have been found between decreased cognition, T2 and T1 lesion volume, and various indices of brain atrophy.
62 –
76 To date, only one study has collected
APOE genotypes, cognitive variables, and quantitative MRI data in the same sample.
77 This was an open-label evaluation of IFN beta-1b in 46 relapsing-remitting patients, of whom seven were ε4+. Although the authors mentioned that
APOE polymorphisms were not associated with cognitive decline or MRI parameters, the omission of important information such as whether ε4+ and ε4− patients were matched on demographic and disease variables renders this report difficult to evaluate, a challenge compounded further by the absence of cognitive and MRI data parsed by ε4 carrier status.
A small literature of seven studies has examined the possible relationship between ε4 carrier status and MR-elicited brain abnormalities in multiple sclerosis.
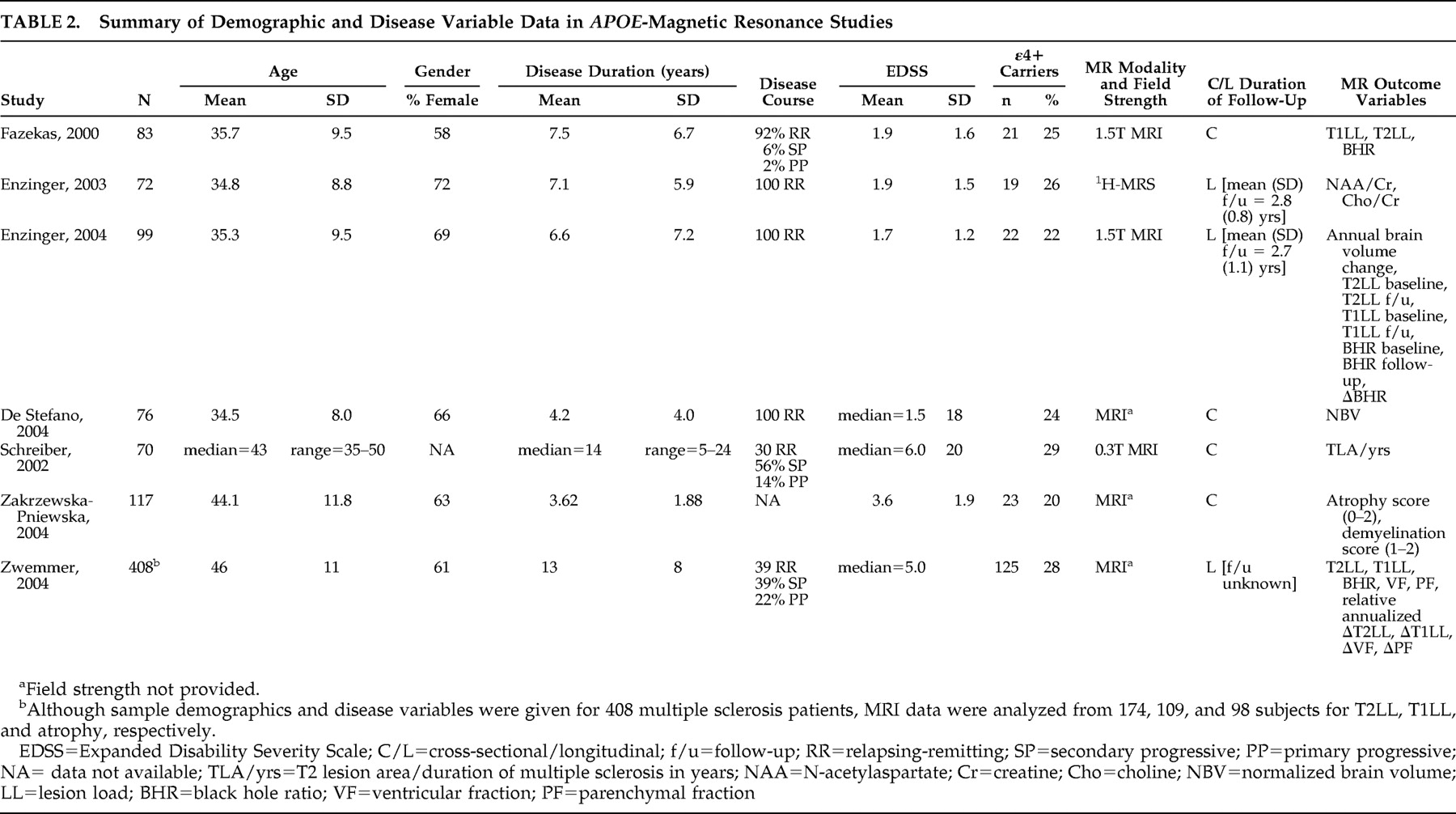
Table 2 illustrates sample demographics, disease parameters, MR modality and field strength, and dependent variable(s) for each report.
Fazekas et al.
78 studied ε4+ and ε4− multiple sclerosis patients matched on age, gender, disease duration, disease course, number of relapses, and EDSS (
Table 2 ). While T2 lesion load (LL) did not differ significantly between the groups, T1LL was significantly higher in ε4 carriers. “Black hole ratio” was also reported, a term defined by: (T1LL/T2LL) × 100. The percentages of black holes in ε4+ and ε4− multiple sclerosis patients were 13.3% and 8.3%, respectively, a statistically significant difference. Given that T1 lesions reflect more destructive tissue pathology, the implication here is that ε4 may be a potential marker of disease severity according to MRI parameters. This is supported by postmortem data linking black holes with axonal swelling and loss, reactive astrocytes, and gliosis.
79 –
82The same group followed up their initial study with a longitudinal proton magnetic resonance spectroscopy (
1 H-MRS) investigation
83 (
Table 2 ). They reported biochemical measures,
N -acetylaspartate (NAA)/creatine and choline/creatine, from a single large volume of interest that comprised parts of the periventricular white matter, the deep white matter, and the basal ganglia. Age, disease duration, EDSS, and treatment with immunomodulatory drugs did not differ between ε4 carriers and noncarriers. At baseline, ε4 carriers showed significantly lower NAA/creatine ratios, a nonspecific marker of neuronal integrity. Two-year follow-up data were available in 44 patients which included 9 ε4+ subjects. The drop in NAA/creatine ratio was significantly greater in ε4 carriers over 2.8 years, while choline/creatine levels, indicative of membrane turnover and/or cellular infiltration, were stable. In a regression model,
APOE ε4 carriage emerged as a significant independent predictor of a low NAA/creatine ratio at follow-up when age at onset, gender, disease duration, and interval between MRS studies were accounted for.
The MRS findings
83 validated and extended the structural MRI data
78 and suggested that ε4 carriage may be independently associated with greater neuropathology, whether it was measured by T1LL or NAA/creatine. Additionally, the drop in NAA/creatine over time in the ε4 carriers suggested that this allele may mediate a faster rate of tissue destruction.
APOE and the dynamics of tissue damage in multiple sclerosis were investigated by the same group in a third study.
84 Rates of brain atrophy, T1LL, and black hole ratios that evolved over a mean follow-up period of 2.7 years were evaluated in ε4+ and ε4− multiple sclerosis patients matched across a host of demographic and disease modifying variables. ε4 carriers had a significantly higher rate of annual brain volume loss and an increase in black hole ratio over the follow-up period. Contrary to their initial study,
78 however, T1LL and black hole ratio—like T2LL—did not significantly differ between the groups either at baseline or at follow-up; it was only the change in black hole ratio over 2.7 years that achieved statistical significance. Regression modeling that incorporated all demographic and clinical variables plus changes in T1 and T2 lesion load and black hole ratio identified the presence of the ε4 allele as the only significant predictor of brain volume changes. Also undertaken was a secondary analysis in which the sample was dichotomized by disease duration above or below 5 years. The findings of the primary analysis—higher annualized brain volume loss and increase in black hole ratio in ε4 carriers—persisted in the cohort with shorter disease duration (n=16) but lost statistical significance in those with disease duration greater than 5 years (n=7), suggesting that atrophy rates in ε4 carriers may be dependent on disease duration.
Smaller brain volumes in ε4+ multiple sclerosis patients were confirmed by De Stefano et al.
85 in ε4+ and ε4− cohorts matched on demographic and disease variables (
Table 2 ). A subgroup analysis looking at patients with short disease duration (<3 years) and relatively low physical disability (EDSS<2) found that the lower normalized brain volumes in ε4+ patients (n=8) compared to ε4− patients (n=22) were evident early in the disease process.
In the studies reviewed thus far, T2LL did not differ between the ε4+ and ε4− patients with multiple sclerosis.
78,
84,
85 Schreiber et al.,
86 using a less sensitive MRI technique (
Table 2 ), were also unable to find a correlation between possession of the ε4 allele and an index of T2 lesions that controlled for disease duration. This report did not examine any other imaging parameters, however.
Zwemmer et al.
87 undertook a retrospective study of 408 multiple sclerosis subjects, of whom 115 were ε4 carriers (
Table 2 ). T2 lesion data, T1 lesion data, and serial atrophy measurements were present for 174, 109, and 98 subjects, respectively. The numbers of ε4 carriers were not given for any subgroups, demographic and disease characteristics were not presented for ε4 carriers and noncarriers, time between scans was not mentioned, and, with the exception of p values, no raw data were shown. Nonetheless, in this largest imaging/
APOE study in multiple sclerosis to date, no MRI differences were reported between ε4 carriers and noncarriers. These findings were unchanged when corrected for age, gender, onset type of multiple sclerosis, duration of disease, and IFN treatment.
The failure to find an association between the ε4 allele and MRI-elicited brain pathology was replicated in a study that had significant methodological problems.
88 MRI scans were evaluated using an “arbitrarily proposed semiqualitative scores system” in which atrophy was scored on a scale of 0 to 2 and demyelination was scored from 1 to 2. The use of nonquantitative, subjective, ordinal scales for MR measures with uncertain floor and ceiling effects is problematic and thwarts critical review of the data.
Comment
Of the seven imaging studies, four support a correlation between ε4 and brain pathology, particularly T1LL,
78,
84 black hole ratio,
78,
84 brain atrophy,
84,
85 and NAA/creatine.
83 These studies had the benefit of sound methodologies that matched ε4+ and ε4− multiple sclerosis patients on demographic and disease variables. Two studies had the additional advantage of a longitudinal study design. However, three of the four studies were conducted by the same research team.
78,
83,
84 While this does not detract from the results, the fact that the subjects were drawn from the same population tempers the generalizability of the findings. Therefore, conclusions regarding an association of the ε4 allele and MR-derived indices of brain pathology cannot be reliably drawn until the above data are replicated in different multiple sclerosis populations.
An interesting addendum to these issues is the potential interaction of ε4-affiliated brain pathology with shorter disease duration.
84,
85 Results here are equivocal. In a longitudinal study, the presence of atrophy was most marked in ε4+ patients with disease duration of less than 5 years.
84 No attempt to control for age was made, however. This finding was not replicated in a second study using a cross-sectional design.
85 Of the two remaining studies that did not find a gene-imaging association, one examined only T2LL
86 and the other had significant methodological problems discussed above.
88