T he most commonly identified agents in cases of sporadic viral encephalitis are herpetic.
12,
13 Although mortality due to herpetic encephalitis has been dramatically reduced by antiviral treatment, the majority of survivors have residual neuropsychological deficits and/or neuropsychiatric symptoms that can be life disabling.
13 –
16 There are presently eight herpetic (from the Greek “to creep”) viruses (herpesviruses) known to infect humans (
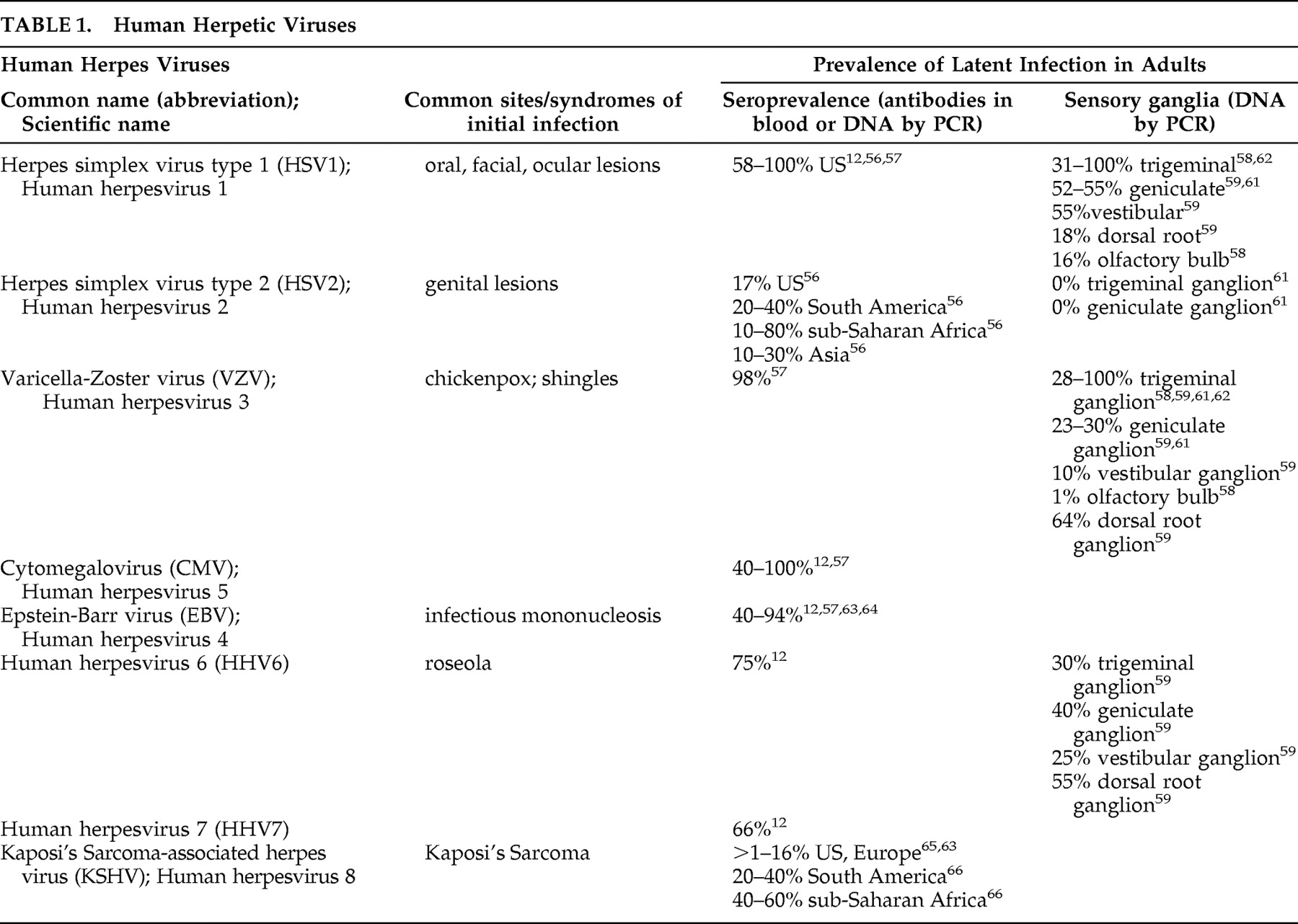
Table 1 ).
17 –
19 All are DNA viruses, requiring the host cell’s DNA polymerase (present in the nucleus) to replicate.
20 DNA viruses thus must enter the host cell in order to reproduce. They are able to remain latent within host cells, with the possibility of reactivation even after very long durations. The herpes simplex viruses (HSV1, HSV2) have been studied the most. Both remain latent in sensory ganglia, with reactivation causing recurrent lesions of the epithelium in the associated sensory distribution (e.g., oral, labial, genital, cutaneous, corneal).
18 There is widespread exposure to this family of viruses, and evidence of latent infection is found in many individuals without clinical symptoms (
Table 1 ).
Entry into the nervous system can occur following peripheral infection.
20 Transneuronal spread has been demonstrated for some herpesviruses, as well as infection of multiple cell types within the brain. Symptoms depend upon the location of the infection. Viral infection of the meninges results in aseptic meningitis (meningeal inflammation), characterized by headache, fever and neck stiffness.
20 –
22 Neurological abnormalities (e.g., seizures, cranial nerve palsies) may also be present. The clinical course is much more benign than for bacterial meningitis, and treatment is primarily supportive. Although most cases of aseptic meningitis (>90%) are due to enteroviruses, some cases have been linked to herpesviruses, particularly HSV2.
20,
21,
23 Myelitis (inflammation of the spinal cord) may affect both sensory and motor tracts as well as neurons within the spinal cord.
20,
22 Infection of the gray matter results in acute flaccid paralysis, often without sensory or autonomic symptoms. Infection of the white matter may cause a transverse myelitis, with disturbances in motor, sensory, and autonomic functions. Herpesviruses are more likely to cause myelitis, and prompt antiviral treatment is recommended.
Encephalitis (inflammation of the brain parenchyma) results when brain cells are infected.
20 Common early symptoms include change in consciousness (confusion, then stupor, then coma), fever, headache, and seizures.
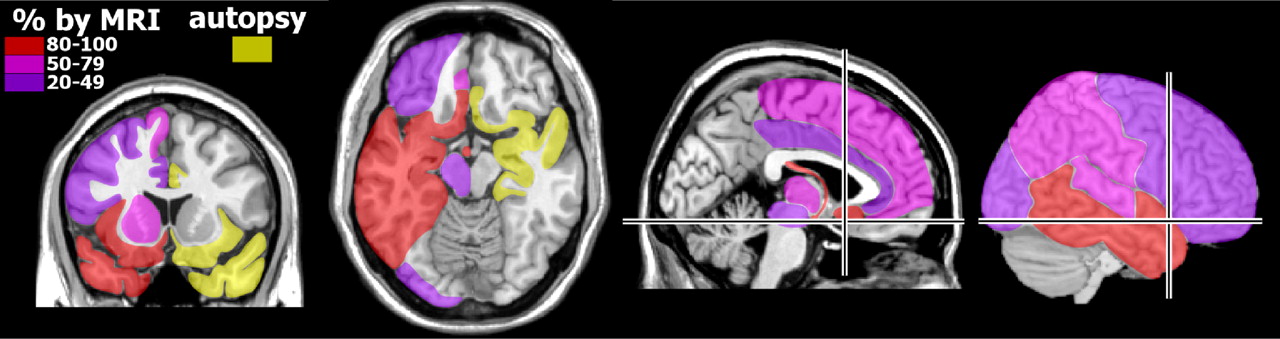
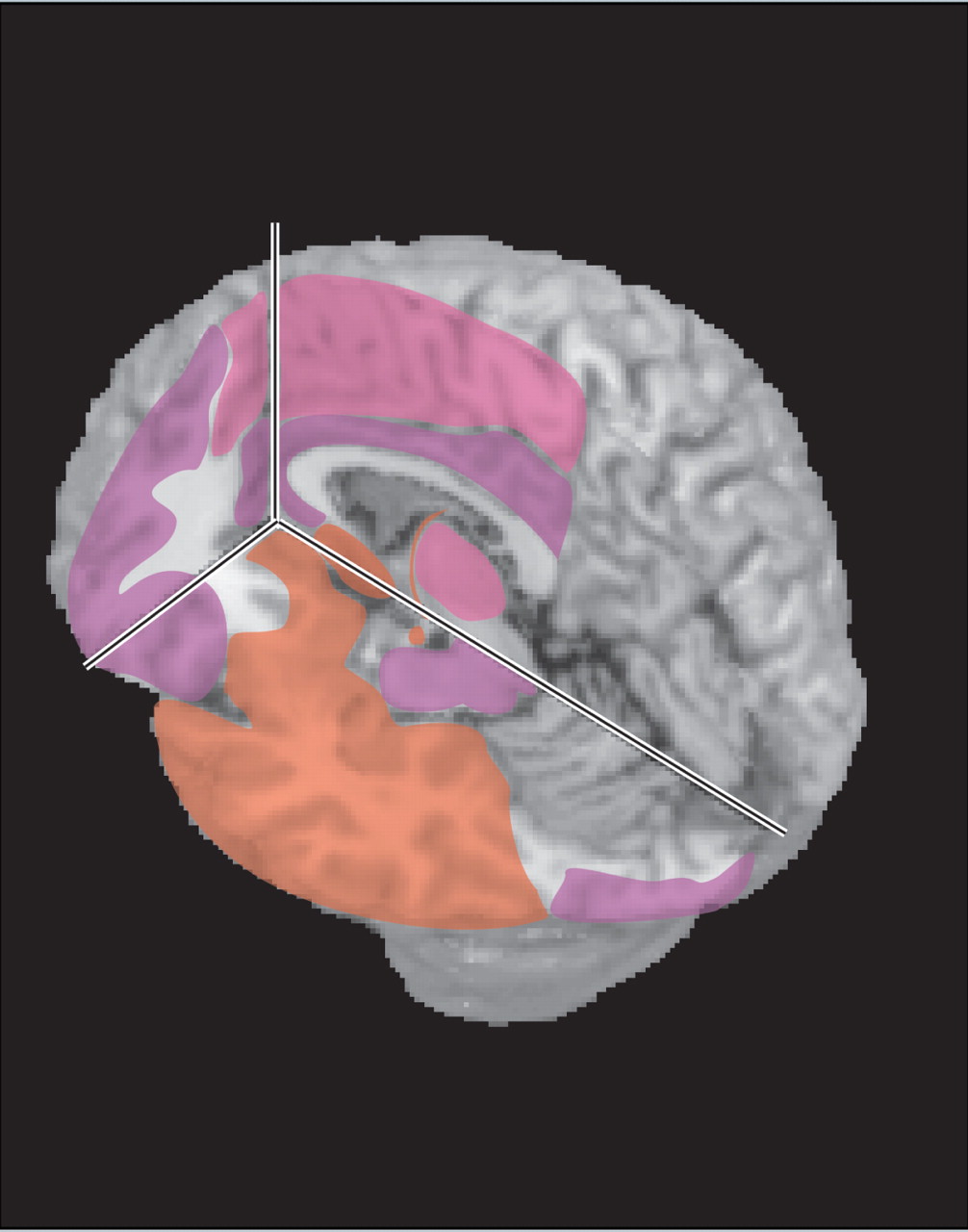
20 Behavioral changes are frequently present, and hallucinations have been reported. The predilection of herpesviruses for limbic-related areas (e.g., hippocampal complex, medial temporal cortex, insula, cingulate cortex) (
Figure 1 ) can help to distinguish herpes encephalitis from other etiologies (e.g., Japanese encephalitis).
5 However, cases without medial temporal lobe involvement at presentation have been reported.
24,
25 Although HSV1 is the most commonly identified agent, multiple reports indicate that the other herpesviruses can cause encephalitis even in immunocompetent adults.
12,
13,
20,
23,
26 –
29Clinical history and laboratory investigations, particularly polymerase chain reaction testing of CSF, are the usual basis for diagnosis.
18,
20,
21 However, polymerase chain reaction may be negative in the acute stage.
7,
21,
30,
31 This is most commonly a necrotizing encephalitis. Delayed initiation of antiviral treatment when a herpesvirus is the causative agent in a patient presenting with acute symptoms, particularly decreased level of consciousness, is associated with higher mortality and morbidity.
14,
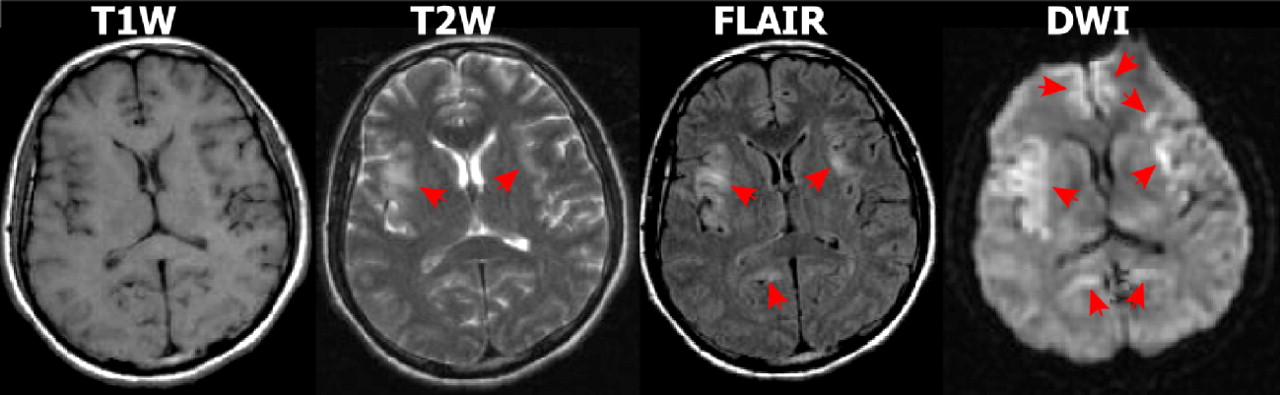
32Multiple case reports and series have suggested that diffusion weighted imaging (DWI) MRI may be more sensitive than other imaging modalities in the early acute stage and thus has potential for supporting prompt diagnosis (
Figure 2 ).
5 –
10,
30,
33 –
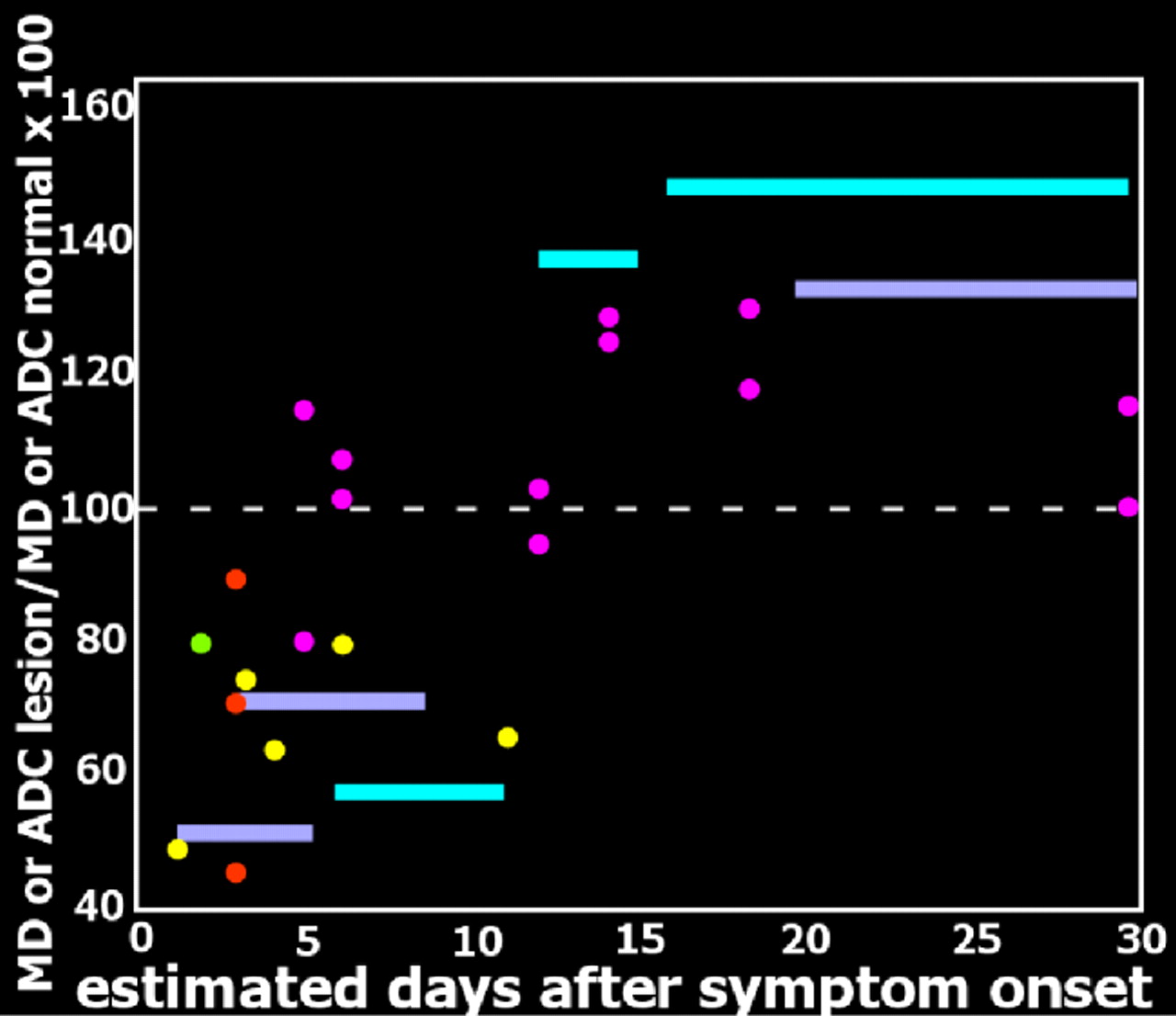
35 In DWI, strong gradient pulses are used to de-phase and rephase the water molecule spins, sensitizing the image to their movement (diffusion) within tissue. Both intracellular and extracellular water movement contribute to imaging appearance. The DWI displays diffusion qualitatively and is also influenced by the T2 of the tissue. Lesions that are bright on T2 weighted images may also be bright on DWI due to “T2 shine through.” It is also possible to calculate the apparent diffusion coefficient (ADC) map, which provides a purer measure. Areas of slow (restricted) diffusion will have high signal on DWI and low signal on the ADC map. Conversely, areas of fast (unrestricted) diffusion will be high signal on the ADC map. The key observation is that the ADC is reduced in lesions in the first few days after symptom onset (
Figure 3 ), resulting in decreased signal intensity on ADC maps and increased signal intensity on DWI, even in the absence of clear changes on T2 weighted (including FLAIR) MRI.
5,
9,
30,
34 At later time points lesions become bright on both T2 weighted MRI and ADC maps (
Figure 3 and
Figure 4 ).
5,
9,
10 Thus in the acute stage diffusion is below normal (restricted), consistent with presence of cytotoxic edema.
36,
37 This progresses to above normal (unrestricted) diffusion at later times, consistent with the development of inflammatory vasogenic edema and necrotic processes.
10,
36,
37It has been proposed that the initially reduced diffusion may indicate cellular swelling as a result of viral intrusion/replication.
10 A neuropathological study of patients who died within a week of symptom onset found very high levels of herpes simplex virus antigen (immunoperoxidase technique) in limbic-related areas with relatively little inflammatory reaction.
1 In most cases, structures in one hemisphere were more severely reactive. Antigen levels were slightly lower in patients who died between 8 and 14 days of symptom onset, and levels were more similar between hemispheres. Areas in which necrosis and edema were evident had reduced antigen. Antigen levels were even lower in patients who died between 15 and 19 days of symptom onset, and more similar between hemispheres, while both necrosis and inflammation were more severe and widespread. No antigen was found in patients who died at later times (22 days–3 years) after symptom onset.
1 A similar progression was reported in animal models of herpes encephalitis, with brain viral load increasing to a peak value at 7 days, then gradually decreasing.
38,
39 It has been reported that lesions within an individual can differ radically in ADC, with some lesions exhibiting slowed diffusion and other lesions exhibiting enhanced diffusion compared to contralateral normal tissue.
8,
36 An intriguing possibility is that these differences reflect lesions at different pathophysiological stages. Sequential infection of areas as a result of viral propagation may underlie this observed difference, as has been suggested based on postmortem immunohistological mapping of viral antigen.
1“The impression gained is of a rapidly spreading wave of viral infection within the limbic structures, probably starting on one side of the brain and spreading within it and to the other side, lasting about 3 weeks and leaving in its wake a trail of devastatingly severe necrosis and inflammation of infected parts of the brain.”
1
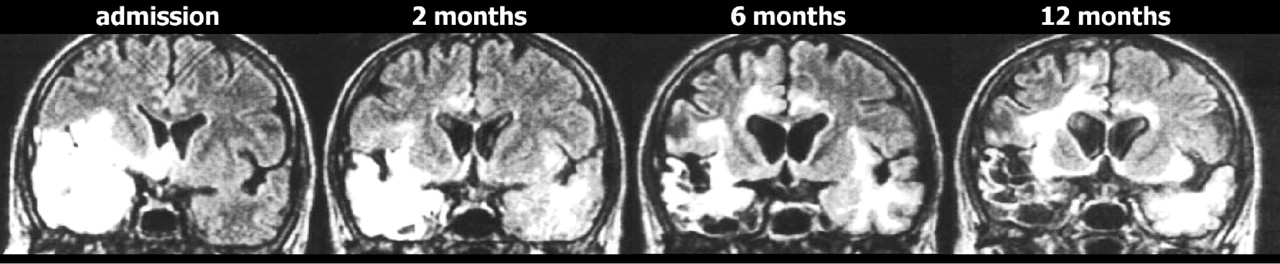
Progressive expansion of lesions on T2 weighted imaging is a common finding in the first few weeks after symptom onset, and is consistent with the expected development of edema, focal hemorrhage and necrosis. Only a few studies have evaluated imaging changes at later times. A few cases consistent with viral reactivation have been reported in both children and adults, with new-onset symptoms and positive polymerase chain reaction.
40,
41 Clinical relapse in the absence of positive polymerase chain reaction has also been reported.
42 As noted by the authors of that study, the possibility of local viral replication in the absence of viral leakage into the CSF must be considered, but the overall picture is more consistent with immunologically mediated pathogenicity. Although herpes simplex virus antigen was not found in brains of long-term survivors (as noted above), herpes simplex virus DNA has been identified in multiple brain areas in long-term survivors including the midbrain and the medulla.
2 The authors of this study noted that this distribution matched the very widespread distribution of inflammatory infiltrate better than the much more localized destructive lesions (temporal, frontal and insular areas). Persistent widespread inflammation has been reported in an animal model of herpes encephalitis.
39 Although the expected concentration of lesions in limbic-associated areas is the most common MRI finding in patients, the presence of abnormalities in many other areas may reflect inflammatory rather than necrotic processes.
4,
5A particularly puzzling pattern has been reported in which clinical symptoms improve but MRI abnormalities continue to progress, with involvement of additional areas including previously uninvolved areas of white matter (
Figure 4 ).
11,
43 –
45 In some cases this has proven to be a self-limiting process, with eventual full resolution.
44 Although viral reactivation is one possible mechanism, the lack of associated clinical symptoms and negative polymerase chain reaction make this less likely.
45 An alternative hypothesis is that these imaging changes are secondary to an immune-mediated process.
43 A similar phenomenon has been documented in an animal model of herpes encephalitis, with a late progressive increase in MRI findings even with acyclovir treatment.
38 Animals treated with methylprednisolone in addition to acyclovir had greatly reduced MRI findings in the chronic stage, suggesting that adjuvant corticosteroid treatment might be clinically useful. A nonrandomized retrospective study found that addition of corticosteroid treatment was associated with better outcome.
46 This is controversial, however, because of the risk that corticosteroid treatment will increase viral spread.
47,
48 A multicenter randomized trial is under way that addresses this important issue.
49The high rate of disability in survivors of herpetic encephalitis supports the need for improved treatment. Although most patients exhibit considerable recovery in cognitive functioning between the acute and chronic stages, long-term impairments are frequently present.
13 –
16,
32,
49,
50 Commonly reported sequelae include epilepsy, memory impairment, personality change, and behavioral abnormalities.
13,
14 Multiple neuropsychological deficits have been found following HSVE, including anterograde and retrograde amnesia, anomie (naming difficulty), executive deficits, and cognitive decline.
4,
13,
16 A recent study suggests that depressive symptoms may be present more frequently than previously thought.
15As is true in other acquired brain injury populations, therapeutic interventions directed toward remediation and rehabilitation are essential for improving patients’ quality of life in the chronic phase. Recent case reports have described useful approaches to particular issues. Examples include adaptation of cognitive behavior therapy to address changes in identity, use of errorless learning paradigms in memory rehabilitation, written versus pictorial diaries to enhance autobiographical memory, and use of automatic reminder systems to improved independence in tasks of everyday living.
51 –
55 There are indications in the literature that encephalitis due to the various herpesviruses may differ in presentation and prognosis. As screening for a wider range of herpesviruses becomes more common, our understanding of these differences will improve. Future longitudinal neuroimaging studies will be crucial to illuminating pathophysiological progression.