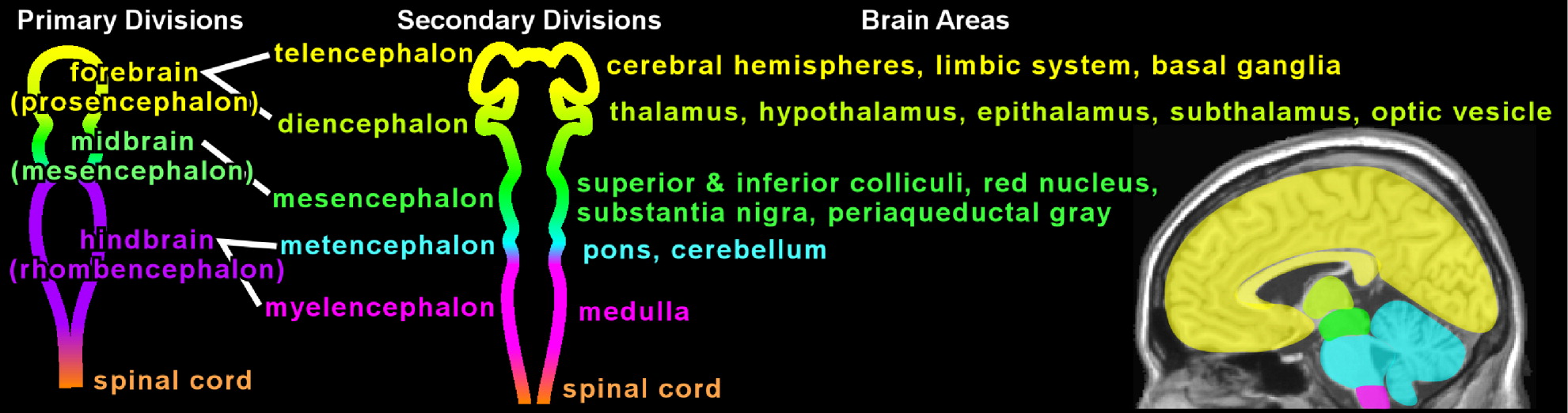
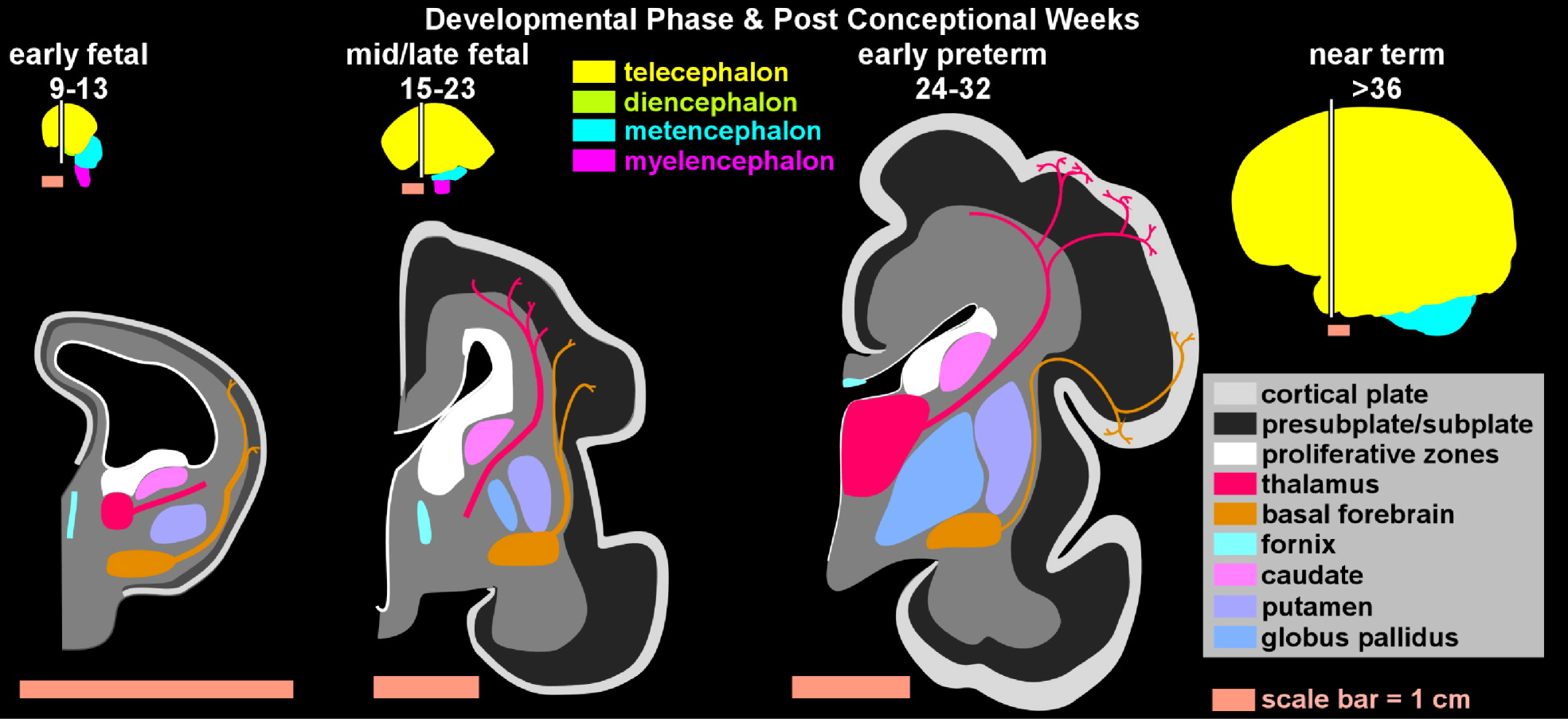
In the last two decades, neuropsychiatric investigation focusing on children and adolescents has begun to offer new paradigms for understanding brain-and-behavior relationships. The medical model archetype, long accepted as fundamental for neuropsychiatric underpinning of disease in adults, is becoming more well developed in pediatric psychiatry. Pediatric imaging studies, although challenged by potential movement artifact and necessary avoidance of radiation exposure, have begun to delineate developmental and structural variability in children.
9 Furthermore, categorical criteria established for psychiatric diagnoses in adults have become more flexibly applied such that they may be relevant to younger age-groups.
10 Identifying symptoms that serve as precursors to illness seen in adults may more efficiently advance understanding of the natural history of disease. Ultimately, it may be intuitive to hypothesize that neuropsychiatric illness identified in adults has anatomic or functional antecedents early in life. In order to fully appreciate the complexities of brain–behavior relationships, it is imperative to understand the developmental stages of the cognitive and emotional pathways in the brain. We present a synopsis of the major pre- and postnatal developmental stages that must occur for normal higher cortical function; these are followed by examples of proposed development abnormalities that lead to pediatric and adult neuropsychiatric disease.
Pediatric Brain Development and Neuropsychiatric Illness
One of the biggest challenges in studying pediatric neuropsychiatric illness is establishing what is normal as a basis for comparisons. The process of childhood development is both variable and unpredictable. Given the numerous tasks of development (e.g., fine and gross motor coordination, language development, logical thought-processing, emotional stability, impulse-control), it is not surprising that the course of normal development is quite heterogeneous. Moreover, many symptoms that would be considered pathologic in adults may be features of normal development in children. For example, young children (toddlers, preschool age) may exhibit aggressive outbursts because of their limited capacity for impulse-control. Modulating aggression by developing verbal strategies of problem-solving is an important task of normal development. School-age children may exhibit separation anxiety in the absence of psychiatric illness as they struggle with self-confidence and self-efficacy in the face of social and academic demands outside of the umbrella of parental influence and security. Latency-age children (age 7–11 years) may exhibit variations of obsessive-compulsive behavior. As children learn cause-and-effect, order, and rules, they may appear compulsively inflexible and rigid when it comes to tolerating transgressions of others or insisting upon “fairness.” Rapidly-changing moods in adolescents may reflect a normal course of emotional maturation, whereas such mood-swings would be considered pathologic later in life.
Despite the wide range of normal behavior, there are some developmental tasks that all humans must accomplish. Brain development is dependent upon neural activity. Synaptic connections develop if neurons are concurrently active; hence, the adage, “Nerves that fire together, wire together.” The most active neurons or neural networks in the brain become more efficient; the least active neurons are likely to be pruned. Experience expectant development refers to periods during which an experience (or its absence) has a marked impact on the neural organization underlying a particular skill or competence. In other words, the brain expects to receive a certain kind of sensory input (e.g., patterned visual information, language) within a critical time-frame (sensitive period). In the absence of appropriate input, neurons of specialized function may not undergo consolidated development, and that function may be permanently absent or impaired. A well-known example is that kittens deprived of visual input in the first 3 months have permanently impaired vision.
16 Finely tuned sound/pitch discrimination appears to be enhanced if children are exposed to a variety of sound pitches before the age of seven.
17 Foreign-language learning that begins in early childhood may be better preserved later in life.
18 One could well conjecture that sensitive periods of development also apply to more complex human functions, such as impulse-control, sustained attention, mood-regulation, and adaptability.
Younger animals' brains are primed to receive information and respond with brain growth much more readily than in older animals' brains. Neurogenesis is particularly enhanced by enriched environments, new learning, and voluntary exercise. It is inhibited by aging, sleep-deprivation, and stress. A recent study assessing the impact of enhanced environments on preterm infants found that the enhanced-environment group had robust improvements in self-regulation, autonomic stability, and EEG normalization. Also, they had MRI findings of more mature tract development in the internal capsules 9 months later.
19 In contrast, the deleterious impact of deprived environments or pathological stress should not be underestimated. Acute stressors trigger physiologic activation (e.g., glucocorticoid release) to ensure the survival of the organism, but to the temporary detriment of systems controlling growth and recuperation. If the brain shifts to a protective mode in order to accomplish self-preservation, then neurogenesis is sacrificed. Frequent activation of the stress response tilts the organism toward consuming resources without sufficient recovery.
20 Severe early neglect affects the development of cortico-limbic circuits involved in emotion and stress responses.
21 Significant anatomic brain changes can be seen in the context of stress and deprived environments. Post-institutionalized children have larger amygdalae, and size corresponds to the duration of institutional care.
22 Furthermore, the neurodevelopmental sequelae of stress may persist over the long term. One study reports that exposure to childhood sexual abuse is associated with a decreased hippocampal volume and a smaller corpus callosum in young adults.
23 However, on a positive note, the physiologic stress-response may be reduced by the presence of a secure attachment figure. Toddlers in secure attachment relationships do not show pathologic elevations in cortisol to distress-eliciting events, whereas toddlers in insecure attachment relationships have higher cortisol levels.
20Although specifying the course of normal brain development is a challenge in studying pediatric neuropsychiatric illness, general principles of brain growth as well as new anatomic MRI studies have led to remarkable insights into variable maturation of different brain regions.
24 Much of brain development involves cycles of cell growth, synaptogenesis, then pruning and remodeling. Cortical cell numbers seem to plateau soon after birth, but the volume of gray matter may increase until puberty, then decrease.
25,26 Formation of connections between neurons and myelination both continue to increase throughout childhood and early adulthood.
27 One of the last regions of the brain to become fully myelinated is the dorsolateral prefrontal cortex, an important region of the brain implicated in tasks of ethical decision-making.
28Perhaps the most notable early-onset neuropsychiatric illness in pediatrics is attention-deficit hyperactivity disorder (ADHD). ADHD is a disorder of impaired impulse-control, distractibility, and psychomotor overactivity. There is evidence that baseline caudate volume is smaller in boys with ADHD than in control subjects, and that it does not decrease in size as would be expected, suggesting impairment in the process of pruning and thus becoming more functionally efficient.
29 Also, one study has reported reversed caudate asymmetry in boys with ADHD, specifically, a smaller left caudate head.
30 The implication is that caudate dysfunction can portend neuropsychiatric illness early in life.
The caudate nucleus also seems to be involved in Tourette's syndrome, another neuropsychiatric illness that usually emerges in school-aged children. Tics are fragments of motor behaviors that escape inhibitory control. Tourette's syndrome may be understood as a model of impaired impulse-control and resultant compensatory response within cortical-striatal-thalamo-cortical circuits. One theory is that the caudate has impaired inhibitory function, and the dorsal prefrontal cortex compensates with increased activity in order to “suppress” tics. If this increased activity leads to a plastic response, the region gets larger. If the plastic response does not occur, it remains small, and more severe tics are observed clinically.
31 However, tic severity in childhood does not predict tic severity in adults. Instead, a smaller caudate volume in childhood predicts tic severity in adults, suggesting that structural imaging in adulthood may be measuring compensatory changes.
32 Typically developing mesial temporal lobe structures such as the amygdala and the hippocampus increase in size through adolescence, and may not mature fully until adulthood.
26,33 In Tourette's syndrome, increases in the dentate and CA3 subfields correlate with symptom improvement. However, increases in the medial body of the hippocampus and posterior surface of the amygdala correlate with more severe symptoms.
34 This may be another instance in which structural-imaging studies done in adults identify compensatory changes rather than abnormalities underlying initial symptom development.
Adult Neuropsychiatric Illness
As described previously, neurons must migrate from the subventricular zone to their final locations to build the cerebral cortex. The machinery required for neuron migration includes cytoskeletal components, with the centrosome serving as an anchor for microtubules that project to the leading edge of the migrating neuron. Once the leading edge is attached (either to the pial surface or to a radial glial scaffold), the contraction of microtubules pulls the centrosome and the nucleus toward the leading process. There is an extensive extra- and intracellular signaling network regulating neuron migration (e.g., reelin/Wnt/Dab pathway, which signals through GSK3β, as well as netrins, semaphorins, slit receptors and integrins). There are also protein complexes at the tip of microtubule and the centrosome that control microtubule polymerization and depolymerization (e.g., Lis1, Ndel, and Disc1).
15 Mutations in some of these genes produce severe developmental brain defects such as lissencephaly.
35Smaller changes in these centrosomic or microtubule-regulatory proteins may be responsible for more subtle defects in cortical architecture, as are seen in schizophrenia. Abnormalities associated with schizophrenia include misplaced and smaller neurons in both cortex and hippocampus in the absence of evidence for neurodegeneration (e.g., no significant gliosis).
36,37 The entorhinal cortex shows similar abnormalities, with decreased cell density and abnormal clustering in layers I/II.
38–40 Molecular markers of neurons, synapses and dendrites (e.g.,
N-acetyl-aspartate,
41 SNAP-25,
42 complexin II,
43 microtubule associated proteins
44) are found at lower levels in multiple brain regions in schizophrenia. There are decreased numbers of GABA-ergic interneurons and interneuron markers in the prefrontal cortex, with more pyramidal cells in deeper layers.
45 NADP-diaphorase also identifies a shifted neuron laminar distribution, with reduced density in superficial layers and increased density in deeper layers.
46,47Neural net simulations have been used to better understand the possible functional results of alterations in cortical architecture. Simulations utilizing the cortical abnormalities found in schizophrenia show aberrant function that is similar to psychotic symptoms.
48 Reduced numbers of parvalbumin-expressing cortical interneurons in schizophrenia diminishes gamma oscillations necessary for proper cortical circuit function.
49,50 This is hypothesized to be a core functional module also disrupted by NMDA hypofunction in schizophrenia.
51 Similar deficits are seen in animal models for schizophrenia.
52 Understanding the mechanisms of abnormal cortical development, and the associated functional impact of the altered cortical histology, is critical to illuminating the pathophysiology of schizophrenia and psychosis, and has the potential to identify novel treatment targets.