Much effort has been devoted to the study of both “normal” age-related cognitive decline and age-related pathology affecting cognition, such as Alzheimer's disease. However, Rowe and Kahn
1 argued that the distinction between normal and pathological is insufficient to describe aging processes, given the heterogeneity found among healthy older adults in various domains, including cognition. Instead, they suggested we further distinguish between “usual” and “successful” aging. Definitions of successful aging vary widely and have included factors such as physical health, cognitive health, life satisfaction and/or well-being, and productivity and/or social activity.
2 Although physical health is commonly included in researcher-defined criteria of successful aging, relatively few older adults who view themselves as aging “successfully” actually meet this criterion.
3 In contrast, unimpaired cognition is a common feature of most researcher-defined criteria of successful aging
2 and a contributing factor named by older adults as important to overall success in aging.
4 Determining factors that promote successful cognitive aging could lead to improvements in the quality of life of older adults.
Research often focuses on what happens “on-average” across a group of individuals, while overlooking variability among individuals. When examining cognitive aging, an “on-average” approach may result in overly simplistic conclusions. Wilson et al.,
5 in a longitudinal study of cognitive function among older adults, illustrate this phenomenon. They found that, as a group, older adults declined in their cognitive performance over time; however, there was great variability among individuals. Whereas some individuals showed steep decline in performance, some showed only gradual decline, others' cognitive performance remained stable, and the rest displayed improvements. This pattern of results exemplifies the heterogeneity in cognitive performance among aging individuals, and it highlights the importance of examining different trajectories of aging.
Individual differences in cognitive performance among older adults may, at least in part, be explained by neurobiological factors such as the size and integrity of brain structures. Some aspects of the relationship between brain structure and cognition in adulthood have been well researched and summarized. Studies examining structural correlates of intelligence in adults have shown that larger brain volumes are associated with higher intelligence scores.
6–8 There is also evidence to suggest that relationships between brain volume and intelligence are genetically determined.
9 Whereas this line of research provides important information regarding volumetric contributions to cognition, age effects are not emphasized. Much is also known about the relationship between brain structure and cognition among older adults with age-related pathology. For example, Alzheimer's disease has been shown to be associated with volume loss in several brain areas, including the hippocampus, parahippocampal gyrus, entorhinal cortex, and the amygdala.
10 Although such evidence suggests that smaller volumes are associated with poorer cognitive functioning in impaired older adult populations, it remains to be seen whether similar relationships are observed as consistently among healthy older adults.
Careful examination of brain–behavior relationships in aging will prove useful in several ways. First, given the heterogeneity in cognitive performance among older adults, at least some of the variability is likely due to differences in brain structure. Determining structures predictive of superior cognitive performance may suggest neuroanatomical correlates of successful cognitive aging. Finally, knowing the relationship between age, brain structure, and cognition in healthy adults might suggest ways in which these factors interact in impaired populations.
Whether brain–behavior relationships change or remain stable across adulthood has not been well studied. Stability in these relationships from younger to older adulthood would support the concept of neural reserve (as described by Stern et al.
11), in that individual differences would seem to persist throughout adulthood, and the more “reserve” an individual has, the greater his or her cognitive abilities. On the other hand, if brain–behavior relationships differ substantially between older and younger adults, this would provide evidence for neural compensation (also described by Stern et al.
11). For example, if a particular brain area is unassociated with a cognitive ability in young adulthood but becomes strongly associated with the ability in older adulthood, one could argue that new brain areas are being used to achieve the same cognitive function in the face of other, negative effects of aging.
In the following review, we sought to examine the brain structural correlates of successful cognition among healthy older adults. We chose to examine cognitive success, in particular, rather than other aspects of successful aging, as cognition is the most widely studied aspect of successful aging in relation to brain structure. We hypothesized that brain structure size would be positively associated with performance in relevant cognitive domains (e.g., hippocampal size and memory performance). Also, we wished to evaluate whether the relationship between brain structure and cognition differed between younger and older adults. Existing reviews
12,13 comment on but do not focus exclusively on some of these issues. Thus, we aimed to address brain structural correlates of successful cognition in a more detailed and comprehensive manner.
METHOD
We conducted a literature review to identify papers in which the relationship between successful cognitive aging and brain structure was examined. A search of PubMed was performed using the following search terms: successful aging OR normal aging OR cognitive reserve AND imaging OR MRI OR computed tomography (CT). Relevant references cited in papers found via this search were also reviewed. This literature search was limited to papers published (or at least readily available in press) before April 1, 2008.
Inclusion Criteria
For inclusion in this review, studies were required to measure age, brain structure, and cognition in healthy older adults. Although factors other than cognition likely contribute to successful aging, we chose to examine successful aging in terms of good cognitive performance (i.e., successful cognitive aging) for the purposes of this review. We included studies that sampled a wide age range (age >18 years), which extended into older adulthood, and studies whose samples consisted entirely of adults over 50 years old. In order to best capture successful aging, only studies of healthy individuals were reviewed, with the definition of “healthy” being left at the discretion of the study authors. Studies of patient populations were also reviewed if a healthy control group was included and if results specific to that control group were reported.
The studies reviewed below had conducted a variety of brain-structure analyses (ranging from whole-brain measurements to measures of specific regions or structures) using MRI or CT. We included studies measuring the volume, thickness, and surface area of brain structures. However, we chose to exclude studies of white-matter integrity and white-matter hyperintensities, believing that their inclusion would result in an overly complicated review and given that a thorough review had recently been conducted including these studies.
14 If a study examined both an included and an excluded brain-structure measure (e.g., volume and hyperintensities), we included it in our review, but we only report findings related to the included measure. There were no specific inclusion/exclusion criteria for measures of cognition.
Review Process
We initially found 485 articles using the above combination of search terms. We then reviewed the titles and abstracts of these articles and identified a subgroup of 34 papers that met our inclusion criteria (listed above) for further review. Sixteen additional articles were obtained from the references cited in these papers. In total, 50 papers met the above criteria and were reviewed. Descriptions of the results from each study are based on the study authors' interpretations of their statistical analyses. We aimed to summarize the relationship of brain structure to cognition among older adults. In addition, we aimed to discuss the available, but limited, evidence concerning whether this relationship is different in younger and older adults. We also report age effects on brain structure. We did not, however, directly examine age effects on cognitive performance, as our focus was on the structural brain correlates of cognition in aging.
RESULTS
Summary information for each study is presented in online Table 1 (with 39 cross-sectional studies) and Table 2 (with 11 longitudinal studies), which are available as online supplements at
http://neuro.psychiatryonline.org/cgi/content/full/23/1/6/DC1.
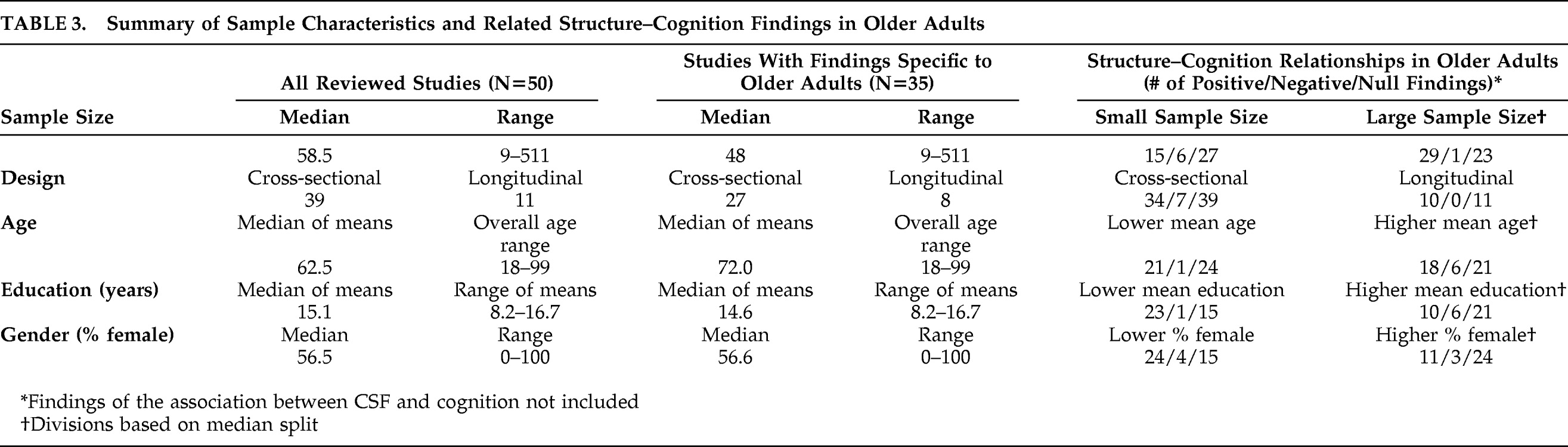
Table 3 contains the demographic characteristics of the samples studied, aggregated across all 50 reviewed papers and across the 35 reviewed papers that specifically addressed structure–cognition relationships in older adulthood.
It was not possible to conduct a meta-analysis of the reviewed studies because of the great methodological variations among them. For example, operationalized definitions of “healthy” used by each study varied from relatively lenient (e.g., no major medical conditions)
15 to relatively strict (e.g., no neurological, psychiatric, or medical conditions; no dementia or signs of MCI in cognitive performance; no evidence of cerebrovascular disease or lesions on MRI; no head trauma with loss of consciousness greater than 5 minutes; not taking any antidepressant, anxiolytic, or antiseizure medications; Mini-Mental State Exam score not less than 26).
16 Domains mentioned as criteria for “health” consisted of following: 1) physical health; 2) cognitive health; 3) psychological health (e.g., no depression or anxiety); and 4) absence of substance abuse/dependence.
Neuroimaging methods and analysis techniques also differed across studies. The vast majority of studies (n=47) collected imaging data via MRI, whereas four used CT scans. In 28 studies, image analysis was done manually, such as hand tracing of a region of interest, whereas eight studies used automated analysis methods, and 13 utilized a combination of automated and manual methods. (One study did not report the analysis methods used). Five investigations conducted both whole-brain and region-of-interest analyses, one exclusively used a whole-brain approach, and the remaining studies used a region-of-interest approach.
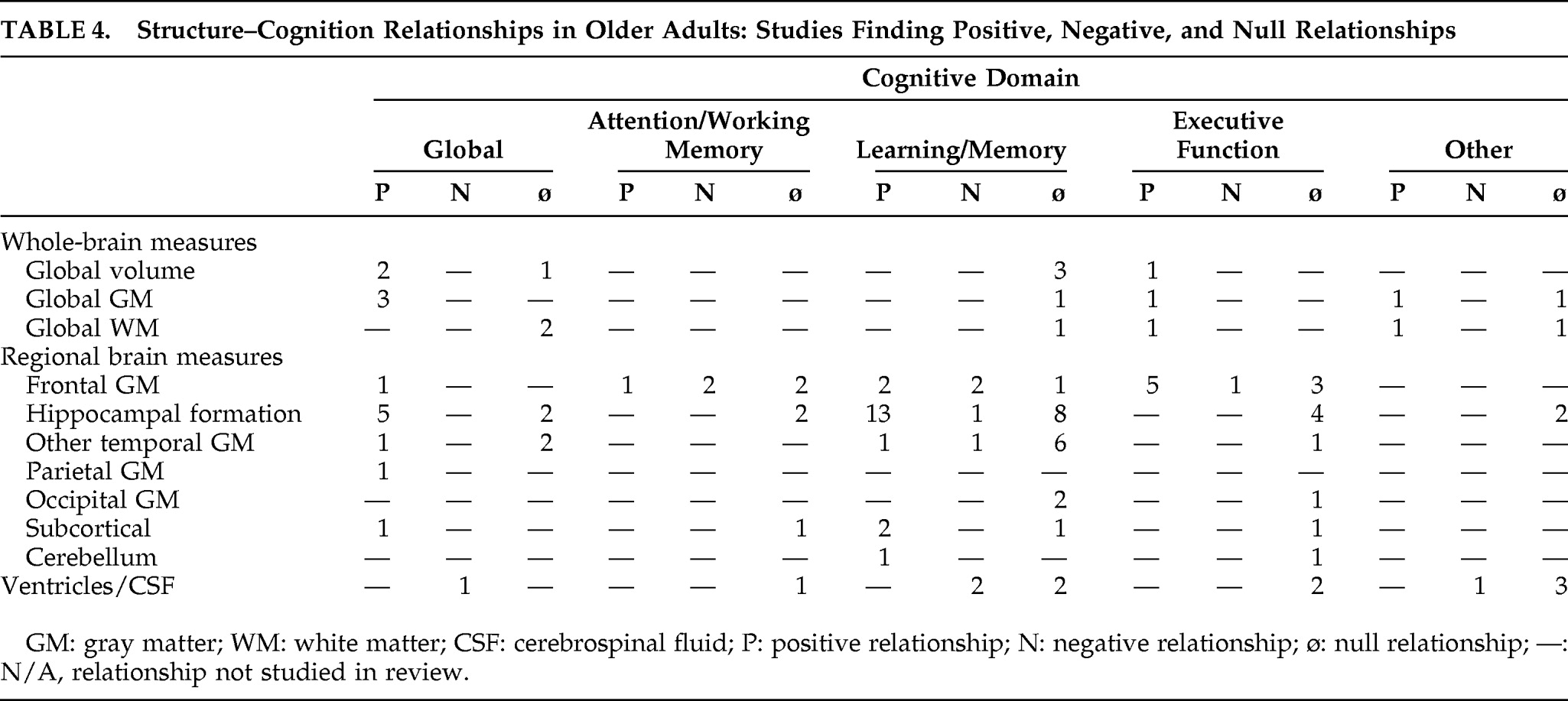
The studies reviewed here examined a wide range of brain measures and cognitive domains. Volume was by far the most common brain measure, collected in 49 studies. Two studies examined cortical thickness, and one measured surface area. Three studies utilized voxel-based morphometry (VBM). Gray-matter regions in the temporal lobe, including the hippocampus, were the most common brain areas measured, followed by frontal brain measures. Although the reviewed studies assessed a wide variety of cognitive domains, memory, attention/working memory, and executive function were emphasized.
Relationship Between Brain Structure and Cognition Among Older Adults
Global Brain Measures
Two of the reviewed cross-sectional studies examined relationships between overall brain size and global cognition among older adults: One found a positive association
17 whereas the other found no relationship.
18 Findings from the only longitudinal study of these factors
19 were consistent with a positive structure–cognition association. When relationships between overall brain size and individual cognitive domains were examined, positive relationships were found with a “frontal” cognitive factor,
20 whereas no associations were found with memory.
18–20Available findings suggest that global gray matter is positively associated with global cognition, both cross-sectionally
21 and longitudinal.
22 Global gray matter was also positively associated with the individual cognitive domains of abstract reasoning and processing speed,
21 and older adults who demonstrated better “fluid” cognitive ability had thicker cortex in several regions.
23 In contrast, global gray matter was unassociated with memory.
21 Unlike global gray matter, the evidence suggests that global white matter is unassociated with global cognition cross-sectionally
21 and longitudinally.
22 However, like global gray matter, global white matter was positively associated with abstract reasoning and processing speed and unassociated with memory.
21Only one study examined the relationship between cerebrospinal fluid (CSF) and global cognition. In this longitudinal study, greater CSF predicted global cognitive decline.
22 Studies more commonly focused on associations between CSF and memory, yielding mixed results. One cross-sectional study found that less CSF was associated with better memory,
24 whereas another found no relationship between these factors.
25 Similarly, one longitudinal study, McArdle et al.,
26 found an inverse relationship, and another found no relationship.
27Frontal Measures
Among studies examining potential relationships between frontal brain measures and cognition among older adults (all cross-sectional), executive function was the domain most often studied. Most evidence supports a positive relationship between the size of frontal structures and executive function. Specifically, positive associations were found for total frontal lobe volume,
28 prefrontal cortical (PFC) volume,
29 and lateral frontal gray-matter volume.
30 Other studies hinted at positive relationships. Namely, Fjell et al.
23 found that “high”-performing older adults did not differ from “average”-performing older adults with regard to cortical thickness, except in a small area in the right middle frontal gyrus. In MacLullich et al.
17 greater frontal volume predicted better abstract reasoning, but only before adjustment for intracranial volume. In contrast, three studies found no relationship between frontal brain structures and executive function, specifically for measures of frontal cortical gray matter,
31 the superior, middle, and inferior frontal gyri,
20 and medial and orbital frontal gray-matter volume.
30 One study found an inverse relationship between executive function and orbital frontal volume.
32Findings regarding relationships between frontal brain measures and other cognitive domains were more mixed. Studies associating frontal measures with attention/working memory performance found positive (orbital frontal volume),
30 inverse (lateral frontal volume,
30 orbital PFC volume
32), and null relationships (total PFC volume,
29 volume of all PFC regions other than orbital PFC
32). Similarly, studies of learning and/or memory also yielded positive (frontal cortical gray matter,
31 lateral PFC
33), inverse (middle frontal gyrus,
20 superior PFC
32), and null associations (frontal lobe volume).
28 Only one study associated frontal measures with global cognition and found a positive relationship with PFC gray matter, longitudinally.
22Temporal Measures
Hippocampus and Related Structures
A positive relationship between hippocampal-formation volume and global cognition was generally supported. Two cross-sectional studies
34,35 and three longitudinal studies
19,22,36 found a positive relationship, whereas two cross-sectional studies found no relationship.
18,37There is relatively strong evidence that larger hippocampal-formation structure predicts better memory performance, as evidenced by the findings of 11 cross-sectional
18,24,34,35,38–44 and two longitudinal studies.
27,36 Nevertheless, this relationship was not universally observed. Four cross-sectional studies
33,37,45,46 and three longitudinal studies
19,47,48 found no association between the structures of the hippocampal formation and memory. An additional cross-sectional study found that greater hippocampal asymmetry (right>left), not total hippocampal volume, predicted better memory.
49 Futhermore, Van Petten et al.,
20 in a cross-sectional study, found an inverse relationship between hippocampal volume and memory.
No significant relationships were observed between hippocampal-formation size and other specific cognitive domains.
20,30,33,44,47Other Temporal Regions
Relationships between cognition and other temporal lobe measurements were explored much less frequently. The most consistent finding that emerged is that temporal measures, other than the hippocampal formation, were unrelated to memory performance (total temporal volume,
46 superior temporal gyrus,
18,24,27,39 fusiform gyrus
39). However, some studies of memory did find significant associations. For example, Lupien et al.
39 observed a positive relationship with volume of the middle inferior gyrus, whereas Van Petten et al.
20 observed an inverse relationship with total temporal neocortical volume, the inferior temporal gyrus, and the fusiform gyrus.
Parietal, Occipital, Subcortical, and Cerebellar Measures
Associations between parietal, occipital, subcortical, and cerebellar brain measures and cognition were rarely studied among older adults. The limited findings included a positive association between posterior parietal cortex and global cognition,
22 null relationships between occipital regions and memory or executive functions,
20 a positive relationship between amygdala volume and memory,
49 and null relationships between amygdala and putamen volumes and attention and executive function.
30 Woodruff-Pak et al.
50 hinted that larger cerebellar volume related to better associative learning abilities, but the relationship was not statistically tested, only graphed.
Summary of Structure–cognition Findings Among Older Adults
Thirty-five of the reviewed studies (27 cross-sectional, 8 longitudinal) addressed potential structural correlates of cognition specific to older adulthood. (The remaining studies did not directly comment on structure–cognition relationships among older adults, often because age was treated as a covariate in samples including younger and older adults). Eighty-three percent of these studies (n=29; n=24 cross-sectional, n=5 longitudinal) found at least one positive association between brain structure size and cognitive performance; however, almost all also found at least one null relationship between a particular brain structure and cognitive domain. In contrast, only 9% of the studies (n=3) commenting on structure–cognition relationships in older adults provided evidence that smaller brain structure size was associated with better cognition.
20,30,32 These were all cross-sectional studies of gray matter, and most of these relationships concerned frontal regions.
Overall, significant structure–cognition relationships emerged more frequently with gray-matter measures and CSF, than with white-matter measures. However, this may be because white matter volume measures were studied infrequently among the reviewed studies. Among the specific brain regions studied, positive relationships between hippocampal formation and cognition (memory and global cognition) and frontal structures and executive function were the most consistent structure–cognition findings. Other relationships that were studied produced inconsistent findings, and many brain structures were sparsely studied.
In order to explore whether the pattern of structure–cognition findings among older adults was related to characteristics of the studied samples,
Table 3 lists the ratio of positive, negative, and null structure–cognition relationships by demographic and other sample differences. Studies with larger sample sizes, lower mean educations, and fewer female subjects appeared to find a higher proportion of positive structure–cognition relationships. Differences in age between the samples and whether or not studies were cross-sectional or longitudinal in design did not appear to affect the ratio of positive, negative, and null structure–cognition findings.
Structure–Cognition Relationships Across Adulthood
While the above findings are important for understanding structure–cognition relationships in older adulthood, they do not address whether these relationships are unique to older adulthood or equivalent to those in younger adulthood. Four cross-sectional studies commented on this issue. In some cases, positive structure–cognition relationships were found among older adults, whereas structure was unrelated to performance in younger adults.
23,30,33 In other cases, the same positive
30,50 or negative
30 structure–cognition relationship held across adulthood. Only one longitudinal study
26 directly addressed this question and found that increases in lateral ventricle size were related to decreases in memory, a relationship that strengthened with age. Of note, there were no findings of stronger structure–cognition associations among younger individuals, as compared with older individuals.
DISCUSSION
The vast majority of studies (83%; n=29 of 35) addressing potential brain structural correlates of cognition in older adulthood suggested that bigger brain structures are associated with better cognitive performance among older adults, at least for some brain regions and some cognitive domains. This caveat is important, however, as most studies that found a significant structure–cognition relationship also found a lack of association for at least one other structure–cognition relationship that was tested. When significant relationships did exist, however, inverse relationships were rare. (The three studies supporting this possibility were cross-sectional in design, and their findings concerned measures of regional gray matter, particularly in frontal cortex). When considered together, the above mixed findings imply that positive structure–cognition relationships exist, but inconsistently at best.
Despite inconsistencies within the findings, some structure–cognition relationships were relatively well-supported. Namely, positive associations were repeatedly observed between hippocampal-formation size and memory and global cognitive performance and between frontal brain measures and executive functions. However, inconsistent findings were evident even for these relationships (similar to those noted in a meta-analysis
51 of hippocampus–memory relationships among older adults). Such inconsistencies may be due to methodological differences between studies, such as variations in sample size, characteristics of the samples, and the particular measures of brain size that were used. Although the vast majority of studies measured brain volume, it is currently unclear whether volume, thickness, or surface-area measures (or some combination of the above) are biologically most relevant for determining cognitive functioning. Stronger and/or more consistent structure–cognition relationships may be found when nonvolumetric measures are more extensively examined. For example, it appears that cortical thickness and surface area may have very different genetic underpinnings,
52 and this may, in turn, cause these measures to relate differently to cognition and show distinct patterns of age-related changes. Also, cognitive measures used to examine structure–cognition relationships may also contribute to inconsistent findings. Because most standardized neuropsychological measures were designed for use in clinical settings, they may not be sufficiently sensitive to detect subtle individual differences related to brain structure in nonclinical populations. Finally, the inconsistent findings may indicate heterogeneity within the older adult population.
Relatively few of the reviewed studies addressed the question of whether structure–cognition relationships in older adulthood are different from those in younger adulthood. Those that did found either equivalent or stronger correlations of brain size with cognitive performance in older than in younger individuals. Because the number of these studies is quite limited, it is difficult to draw strong conclusions from them or to find patterns within them that explain why some showed equivalence and others showed stronger correlations among older participants. It is notable, however, that no studies found evidence for stronger relationships in younger individuals. This lack of findings argues against the idea that experience and/or cognitive strategies gained with age might attenuate the relationship between brain structure and cognitive performance.
Additional longitudinal research examining structure–cognition relationships across the adult lifespan is necessary in order to better understand the neural factors associated with successful cognitive aging. Given time and cost limitations of traditional longitudinal designs, an accelerated lifetime design, in which subgroups of individuals of overlapping age-groups are followed, could best reveal the trajectory of change over a large age-span. This research is needed because it is currently unclear whether individual variability in brain structure size merely persists into old age, leading those with larger structures to perform better cognitively, or whether there are neural changes that occur with age that promote successful cognitive aging. As previous findings suggest that experience can produce brain structural changes,
53,54 it is possible that interventions could be developed to facilitate successful cognitive aging through neural mechanisms.
Future research on successful cognitive aging would also benefit from standardization of the definition of “health,” with careful consideration of screening for mild cognitive impairment (reported by only one study in the review). Also, since the brain regions examined in the reviewed studies were somewhat limited, future studies should expand consideration to other structures perhaps based on genetic or developmental evidence suggesting that they form larger, functionally relevant structural units within the brain. Finally, a complete understanding of the neurobiological underpinnings of successful cognitive aging will likely require examination of both brain structural and functional measures and their interaction.
55There are several limitations to our review to consider when interpreting our findings. First, we may have failed to include some studies that met our inclusion criteria. Our review is also likely biased toward reporting significant structure–cognition relationships, as studies that do not find significant relationships are less likely to be published. Furthermore, our summaries of the reviewed studies are somewhat limited with regard to their level of detail because of the number, complexity, and diverse methodologies of the reviewed studies. Also, although our review describes relationships between brain structure size and cognition, it does not indicate what these relationships might mean on a neurobiological level. We also did not include findings from DTI studies of white-matter integrity (see Sullivan and Pfefferbaum
14 for a thorough review of the age-related links between cognition and white-matter integrity), and a recent paper not included in our review, Ziegler et al.,
56 suggests that associations between cognition and white-matter integrity may be stronger than those with gray-matter measures, such as cortical thickness. Thus, stronger and more consistent relationships between brain structure and cognition may emerge among older adults when white-matter integrity is considered. Finally, because successful aging is a broad concept without a consensus definition, our focus on cognitive performance means that the results of this review speak only to one aspect of successful aging. Indeed, results would likely differ if another aspect of successful aging (e.g., emotional well-being) was examined in relation to brain structure.
Research on the brain structure correlates of successful cognitive aging is a promising area of inquiry that has already received much attention in the literature. Research to date suggests positive structure–cognition relationships, particularly for the hippocampal formation and frontal lobe; however findings are inconsistent, at best. Further research is needed especially regarding whether the relationship between brain structure and cognition strengthens with age, thereby shedding light on how the processes of neural reserve and neural compensation might contribute to successful cognitive aging and perhaps suggesting when and how to intervene in order to enhance cognition in old age.
Acknowledgments
This work was supported, in part, by the Sam and Rose Stein Institute for Research on Aging, the NIMH (T32 Fellowship MH019934), the Advanced Center for Innovation in Services and Interventions Research (P30 MH080002), the Vietnam Era Twin Study of Aging (VETSA) MRI Study (AG01834, AG01836, and AG022381), and the VA.