Brain oscillations in specific frequency bandwidths reflect the arousal of functionally-distinct neural systems.
1 Low-frequency rhythms (delta, theta) originate in more medial and primitive brain regions than high-frequency activity (beta, gamma), which reflects oscillatory properties of the neocortex.
2 Recently, some investigators have suggested that cross-frequency interactions observed in evoked
1 and spontaneous
2 oscillations reflect relations among different neuroanatomical levels. Spectral coupling between delta and beta frequency bands provides an electrophysiological correlate of cortico–subcortical cross-talk
3 that is sensitive to motivational states and neuroendocrine patterning. Frontal brain delta–beta coupling is increased in relation to high cortisol, behavioral inhibition, and anxiety.
4–6 By contrast, testosterone, an anxiolytic hormone, is associated with diminished coupling.
5,7Because perturbations of frontal cortical–subcortical circuits are implicated in psychiatric disorders,
8 this may be reflected in brain oscillations that putatively index some of these neurophysiological processes. We have recently shown that adults with social anxiety disorder exhibit strong delta–beta coupling that is reduced by cognitive-behavior therapy.
9 However, no studies to-date have examined cross-frequency interactions in first-degree relatives of individuals with anxiety disorders, despite behavioral evidence that children of socially phobic parents exhibit increased psychiatric impairment.
10The purpose of the present pilot study was to examine resting frontal brain oscillatory coupling in children born to socially phobic versus healthy parents. We hypothesized that the offspring of socially phobic probands would show stronger delta–beta coupling than the offspring of control subjects.
METHOD
Participants
Children of Socially Phobic Parents
We recruited six right-handed children (three boys) who ranged in age from 7 to 14 years old (mean: 10.67 years; standard deviation [SD]: 2.88) and who had a biological parent with a primary DSM–IV diagnosis of social phobia. Their parents were identified from the current patients at the Anxiety Disorders Clinic, Hamilton Health Sciences, at McMaster University Medical Centre. The authors recruited a random sample of patients from the Anxiety Disorders Clinic on the basis of their willingness to participate. All patients (N=5) were given a structured DSM–IV interview;
11 four patients had diagnosis of generalized social phobia, and one patient had nongeneralized social phobia. It is important to note that one parent had two children participating in the study. All but one parent had at least one comorbid diagnosis. The structured clinical interview was performed by an experienced clinician.
Children of Healthy Parents
We selected 10 typically-developing right-handed children (5 boys), with a mean age of 10.25 (SD: 1.04) years to serve as a comparison group. These children were chosen from a child database in the Department of Psychology, Neuroscience, and Behavior containing the birth records of healthy children born at McMaster Medical Centre. All the comparison children were Caucasian and from middle-income households. All comparison children and their parents were healthy and free from psychiatric and neurological illness and family history of psychiatric problems.
Importantly, children of socially phobic and comparison parents did not differ on age, race, socioeconomic status, and handedness variables. The children in the two groups did not differ in age or gender composition.
Procedures
Informed consent was obtained for all participants and their parent(s) before we began any procedures. After the child had a chance to acclimate to the lab, he or she was directed into a testing room, seated in a comfortable chair, and the EEG cap was placed on his or her head. The child was instructed to relax and to keep movements to a minimum, but not to be overly concerned about doing so. During this time, the parent was in an adjacent room completing the Shyness subscale of the Colorado Childhood Temperament Inventory.
12 All procedures were conducted under the supervision of trained research staff and approved by the McMaster University Health Sciences Research Ethics Board.
EEG Data Collection
Regional EEG data were collected during a 2-minute resting condition (1 minute eyes-open; 1 minute eyes-closed), using a fabric stretch-cap. The cap electrodes were positioned according to the 10/20 system of the International Federation. EEG was recorded in the left and right anterior and posterior regions (F3/F4, C3/C4, P3/P4, O1/O2) referenced to common vertex (Cz). Impedances below 10 K ohms for each electrode site were considered acceptable. Channels were amplified with SA Instrumentation Bioamplifiers, and bandpass was filtered between 1 and 100 Hz. The EEG data were digitized online at a sampling rate of 512 Hz.
EEG Reduction and Analyses
The EEG data were visually scanned, and all artifact due to movement (e.g., eye blinks, body movements) was edited out, using software developed by James Long Company (EEG Analysis Program, Caroga Lake, NY). If an artifact was present in one channel, then data in all channels were excluded. All artifact-free EEG data were analyzed with a discrete Fourier transform, with a Hanning window of 1 sec width and 50% overlap. The groups did not differ in the amount of artifact-free EEG data, expressed as a ratio of discrete Fourier transform windows to epoch duration (p>0.34). Following the pattern of landmark EEG studies with similar age-groups,
13 we computed spectral power (μV
2) in delta (0 to 4 Hz) and beta (12 to 17 Hz) bands and performed a natural log-transform (
ln) to reduce skewness. Because power in eyes-open and eyes-closed conditions was highly related (p<0.01), the two conditions were combined to form a composite measure of resting EEG power separately for each region.
RESULTS
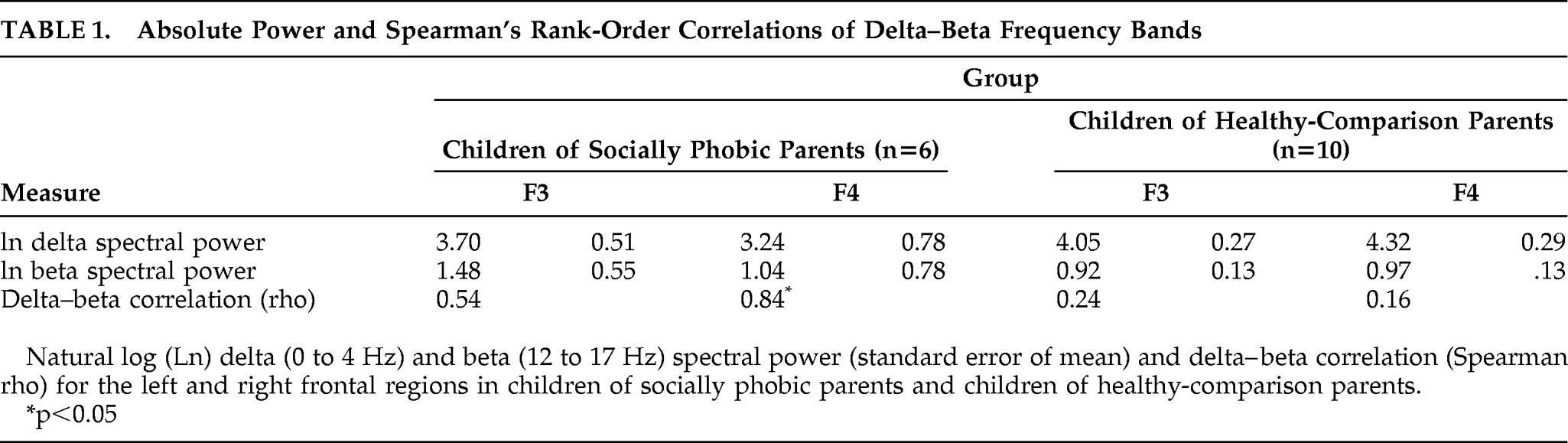
Given the small sample size of the groups, we used nonparametric statistics to test our main hypotheses. Delta–beta coupling was estimated with Spearman's rank-order correlations. As shown in
Table 1, children of socially phobic parents showed stronger coupling than children of healthy parents in both the left and right frontal leads. In order to test for group differences in the magnitude of coupling, we converted the coefficients to
z scores, using Fisher's Z transform and then used an independent-groups comparison.
As predicted, children of socially phobic parents exhibited significantly greater delta–beta coupling in the right (Z=2.00; p<0.05), but not the left (p>0.60), frontal lead, as compared with children of nonphobic parents. The effect size for the between-group difference in right frontal coupling was large (q=1.38; calculated as the difference between Fisher's Z scores [q=Z1−Z2]). There were no differences in posterior (parietal, occipital) brain oscillatory coupling (p values >0.40).
By contrast, there were no significant group differences in absolute spectral power of either EEG bands in any of the regions (p>0.14 in all cases), suggesting that the groups differed specifically in the magnitude of coupling. Finally, there were no significant differences in parental report of shyness (p>0.90), between the children of socially phobic parents (mean=12.18 [SEM=0.95]) and children of healthy comparison parents (mean: 12.09; [standard error of mean: 1.31]).
DISCUSSION
The results of this pilot study provide preliminary evidence that offspring of parents clinically diagnosed with social phobia differed in patterns of oscillatory EEG coupling during the early school-age years when compared with children of healthy parents. Specifically, children of socially phobic parents exhibited increased frontal coupling of delta and beta oscillations, which is related to elevated anxiety, behavioral inhibition, and cortisol concentrations.
4–6 We speculate that these effects may have been stronger in the right frontal region because of increased anatomical connectivity of the right cerebral hemisphere with limbic structures.
14 Enhanced delta–beta coupling may be an easily-quantified endophenotype that reflects functional alterations in the cortico–subcortical circuitry of children born to socially phobic parents that makes them more vulnerable to future psychiatric impairment. It should be noted that the groups did not differ in parental report of shyness, possibly because of the early age of assessment. Indeed, it has been shown that children of socially phobic parents exhibit an increase in anxiety disorders during the transition into adolescence and young adulthood, a developmental period when social challenges are particularly salient.
10We urge caution in interpreting the results of this study, given the very small sample size and the preliminary nature of the data. Also, no conclusions can be drawn about whether the brain oscillatory differences between the two groups reflect genetic or environmental contributions. It is possible that children of socially phobic parents present with altered EEG oscillatory patterns due to parenting rather than, or in addition to, inherited differences in neurophysiology. Also, it should be emphasized that the interpretation of delta–beta coupling as reflecting cortico–subcortical dynamics is at present best viewed as a heuristic model, until there is converging evidence from neuroimaging.
Future studies should examine offspring of parents with psychiatric disorders and should use larger sample sizes and multiple measurement occasions across the course of development. Also, studies should collect information on the quality and styles of parenting and other sociodemographic factors in order to clarify the separate or interactive effects of biology and environment.
Acknowledgments
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Social Science and Humanities Research Council of Canada (SSHRC) awarded to Louis Schmidt and a Vanier doctoral fellowship from NSERC awarded to Vladimir Miskovic under the direction of Louis Schmidt.