Previous research suggests that Parkinson's disease (PD) is associated with particular personality traits, the so-called parkinsonian personality. Patients with PD have been described as unassuming, loyal, cautious, deferential to authority, rigid, and less novelty-seeking than healthy elderly persons.
1,2 These traits may even be present premorbidly because of early degeneration of dopamine networks, and the traits then persist or increase in severity after disease onset.
2,3 However, the existence of a unique parkinsonian personality is still controversial, and others have found no significant differences in personality profiles between PD and medical control subjects
4–6 or twin control subjects discordant for PD.
7A possible explanation for the different results on the parkinsonian personality could be depression acting as a confounding factor. The relationship between concomitant depression and the parkinsonian personality has not been fully explored, but recent research suggests that PD with depression is associated with increased Harm Avoidance on the Cloningers Tridimensional Personality Questionnaire.
8,9 Hence, Jacobs et al.
10 suggested that the difference in Harm Avoidance between PD and control subjects could be explained by the 56% depression prevalence rate in their PD group. Similarly, Fujii et al.
11 stated that the increased Harm Avoidance and decreased Novelty-Seeking in their PD group could be related to concomitant depression (as indicated by the use of antidepressants).
The present study was designed to further elucidate the relationship between concomitant depression in PD and personality by directly comparing the personality profiles of depressed PD patients with healthy-control participants and PD patients without depression.
METHOD
Participants
A total of 480 outpatients with PD, residing in the region formerly known as Aarhus County, Denmark, were identified from a review of medical records, at the University hospitals of Aarhus. Some patients were identified by neurologists practicing in the county. PD was diagnosed in accordance with the United Kingdom Parkinson's Disease Brain Bank Diagnostic Criteria.
15A total of 272 patients were excluded for the following reasons: dementia (N=53), severe psychiatric illness other than depression (N=7), other neurological disorders than PD or head trauma with loss of consciousness (N=76), alcohol or substance abuse (N=5), moved outside the county or had Danish as a secondary language (N=15), had deep brain-stimulation implant (N=20), were under age 50 at disease onset (N=60), suspicion of other diagnosis than idiopathic PD (N=36).
The remaining 208 eligible patients were offered participation in the questionnaire study; 129 patients returned the questionnaires; 49 patients did not wish to participate; and 30 patients did not respond. The overall response rate was 62%. A further 11 patients were excluded from the study because of incomplete questionnaires. A total of 44% of the participants were men. None were taking antipsychotic medication. A total of 22 patients were being treated with antidepressants; 5 receiving tricyclic antidepressants, and the remaining 17 selective serotonin reuptake inhibitors. Seventy of the patients also participated in a still-unpublished neuropsychological study within 5 months of the questionnaire date (mean: 1.24 months; standard deviation [SD]: 2.17 months), where all had a Mini-Mental State Exam (MMSE) score above 24 (see
Table 1). Of the remaining 48 patients, 15 had MMSE scores recorded in the medical records, and all were seen by a neurologist within 13 months of the questionnaire date (mean: 5.13 months; SD: 3.75 months). None was diagnosed with dementia or treated with antipsychotic medication at this time.
Thirty healthy-control participants (13 men) were recruited by advertisements. They were all community residents without any neurological symptoms, severe chronic physical disease, or psychiatric illness. None had substance or alcohol abuse, or were taking medications with effects on the CNS. None received domiciliary care. All control participants also took part in a still-unpublished neuropsychological study and had an MMSE
16 score ≥27 (mean: 29.10; SD: 0.22).
Procedure
The study was reported to the Danish Data Protection Agency and was carried out in accordance to the Declaration of Helsinki. All participants received a letter explaining the purpose of the study, a consent form, the questionnaires, and a prepaid envelope by mail. Nonrespondents received one reminder after 2 weeks. All participants were asked to fill out the questionnaires themselves and to double-check all answers before returning them. Hence, caregivers were not allowed to fill out the questionnaires on behalf of the patient.
Measures
Demographics and Health
Details on age at disease onset, general health, motor symptoms, medications, Hoehn and Yahr stage,
17 MMSE (when available), and disease duration were recorded from medical records. Further information, on motor symptoms, motor fluctuations, and other symptoms were obtained from the participants via a questionnaire consisting of 1) a general health section (How would you describe your health? Do you take any medication for other diseases or ailments than PD? If yes, which? Do you receive domiciliary care?); 2) a PD symptom section (When did you first experience symptoms of PD [year]? When was the diagnosis made by a neurologist [year]? Where did the symptoms first appear [right arm, right leg, left arm, left leg]? Where are the dominant symptoms now [right arm, right leg, left arm, left leg]? Which of the following symptoms have you experienced within the last 30 days [I experience tremor or shakiness while resting; for instance, sitting with my hands in my lap? I experience tremor or shakiness while writing or doing other movements? Sometimes I “freeze” and have difficulties moving? I have difficulties keeping balance and tend to fall? I have noticed that my movements have become slower? I feel stiffness or rigidity in my muscles? I have involuntary big movements [dyskinesias] that are not tremor? Sometimes my medication stops working and I have trouble moving?); 3) a PD medication section (I would describe the effect of my medication as: good, moderate, limited, or poor? It is predictable when the effect of my medication wears off: yes, no, or it wears off in seconds? Have you experienced any of the following side effects in the last 30 days: nausea, vomiting, or loss of desire to eat? Sleeplessness, tiredness or other sleep disorders?).
The Short Geriatric Depression Scale (GDS–15)18
Severity of depressive symptoms was assessed with the Danish version of the GDS–15.
19 The GDS–15 is a self-administered, 15-item questionnaire especially suitable for PD patients because it contains no somatic items.
20 A cutoff score of 5 indicates clinically important depressive symptoms and has a sensitivity of 91% and a specificity of 72% when compared with validated psychiatric diagnosis.
21The NEO Personality Inventory, Short Version22
The Danish version of the NEO-Five Factor Inventory was used to assess five personality dimensions as derived from the five-factor model of personality. The five personality dimensions assessed are Neuroticism (tendency toward experiencing psychological distress or negative affectivity), Extroversion (tendency to be sociable, novelty-seeking, and adventurous), Openness (openness to internal and external stimuli, including imagination), Agreeableness (trustfulness, altruism, modesty), and Conscientiousness (self-discipline and competency). Importantly, the questionnaire assesses traits (stable characteristics of personality) as opposed to states (temporary psychological states and moods).
The questionnaire consists of 60 statements that the respondents rate on a 5-point Likert scale from “strongly disagree” to “strongly agree.” The NEO-PI–R Short Version is a shortened version of the 240 item NEO-PI–R. However, correlations of 0.89–0.93 between the short and long version of the questionnaire have been reported.
23 Validity and reliability of 0.73–0.84 have been reported for this scale.
22Statistical Methods
Data were analyzed with SPSS 17.00. The patient group was subdivided into a Depressed (PDd; with GDS score ≥5; N=32) and a Nondepressed (PD; GDS ≤4; N=86) group; t-test and chi-squared test were used to compare the two groups on clinical and demographic variables. One-way, between-group analysis of variance (ANOVA) was used to compare the PD, PDd, and control groups on demographic variables. Multiple analysis of covariance (MANCOVA) was applied to compare the personality profiles of the PD, PDd, and control groups, with age and years of education as covariates. Also, a MANCOVA was performed using disease features as covariates. Preliminary assessments were performed to ensure that assumptions of normality, homogeneity of variance, linearity, and homogeneity of regression slopes were not violated. Whenever a significant main effect was found, group differences were tested by one-way, between-groups ANOVA, with Tukey's honestly significant difference [HSD] post-hoc comparison procedures.
To ensure the representativeness of the control-group, scores on the NEO-FFI were initially compared with the existing Danish normative material supplied with the questionnaire, and the scores were deemed comparable.
24RESULTS
The PD and control group did not differ significantly in terms of age (PD mean: 71.29 (7.45) years, and control mean: 69 (5.73) years; t[54.36]=1.840; p=0.071) or in years of higher education after municipal primary and lower secondary school (PD mean: 3.81 (2.81) years, and control mean: 4.37 (2.77) years; t [157] = –0.973; p=0.332).
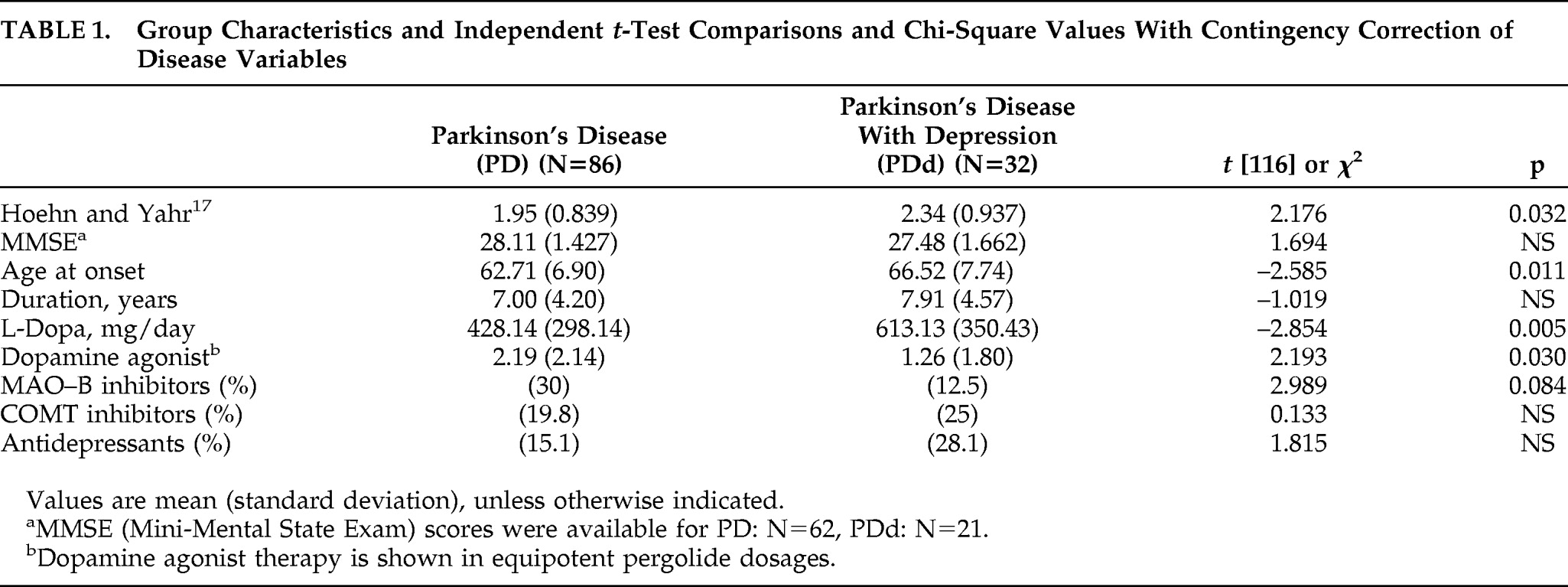
Clinical and Demographic Variables
A chi-square test with contingency correction showed no significant gender difference between the PD (55 men) and PDd group (31 men); (χ
2[1]; N=118)=0.347; p=0.556; Φ = –0.074). Group characteristics and comparisons of disease variables are shown in
Table 1. The PDd group was significantly older at disease onset, had greater disease severity, and received significantly larger
L-dopa doses than the PD group. The PD group received significantly more dopamine-agonist therapy than the PDd group. There were no significant differences in disease duration, MMSE score, use of MAO-B inhibitors, COMT inhibitors, or antidepressants between the two PD groups.
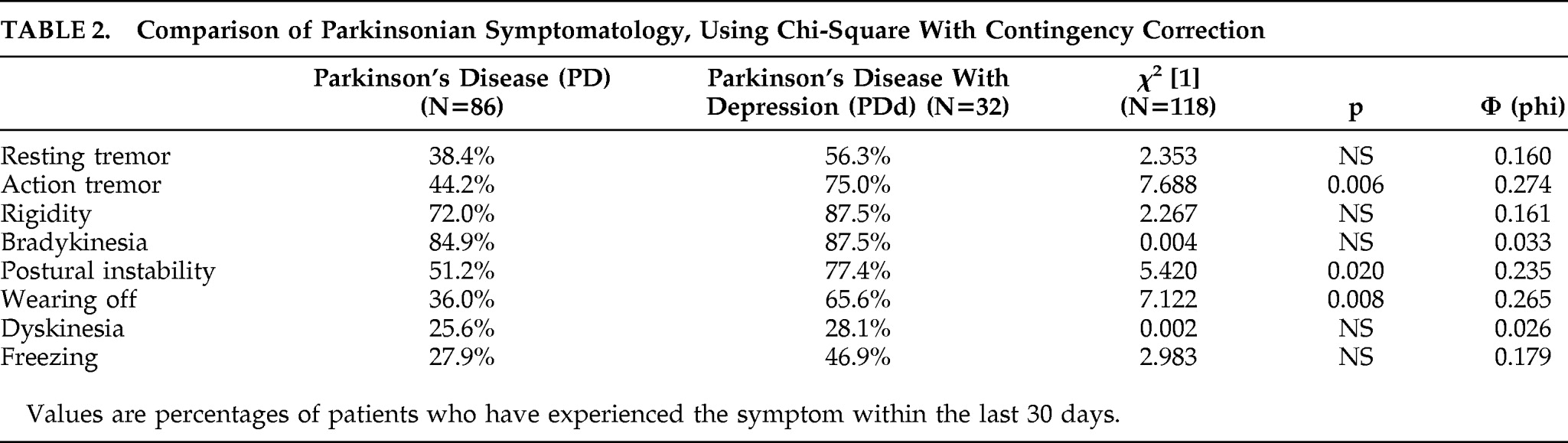
The symptomatology of the two PD patient groups was further compared, using a chi-square test for independence (with Yates Contingency correction). As shown in
Table 2, the PDd group experienced significantly more action tremor, postural instability, and wearing-off episodes than the PD group. There was no significant difference between the two PD groups on resting tremor, bradykinesia, rigidity, freezing, or dyskinesia.
The PD and PDd groups were compared with the control group on age and education by use of one-way ANOVA. Significant age differences were detected between the three groups (F[2, 145]=6.51; p=0.002). Post-hoc Tukey's HSD comparisons showed that the mean age for the PDd group (74.43 [7.54] years) was significantly higher than for the PD group (69.61 [7.10]) and the control group (69.02 [5.73]). The control and PD group did not differ significantly with regard to age. There was no significant difference in years of education among the three groups (F[2, 145]=0.491; p=0.613).
Personality Measures
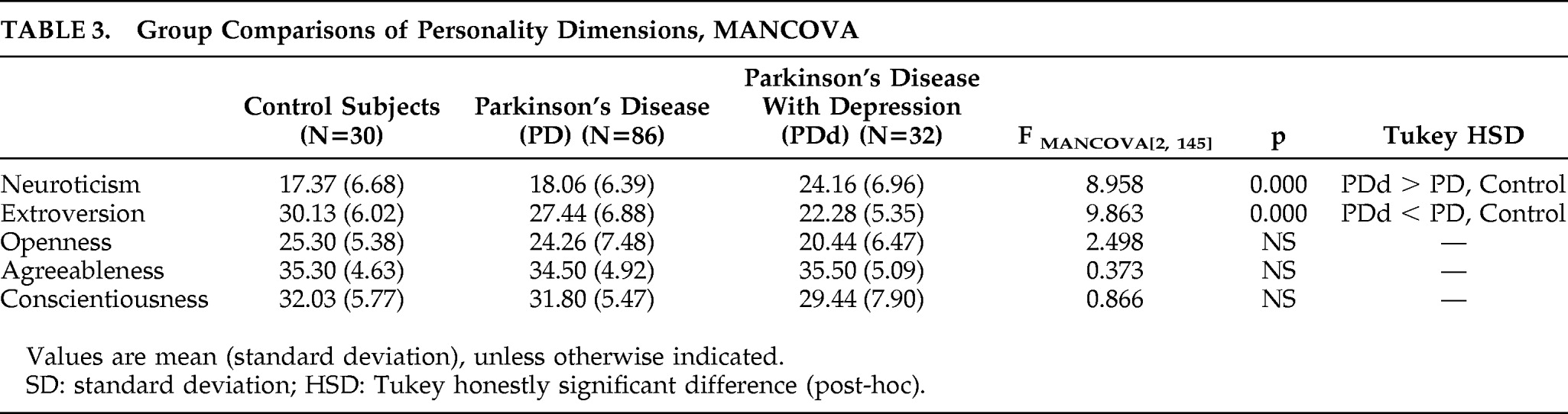
MANCOVA was applied to assess differences in personality dimensions among the PD, PDd, and control groups. Because demographic characteristics of age and education have been found to affect personality, all analyses were performed with age and years of education as covariates. As shown in
Table 3, there were no significant differences between the three groups on Agreeableness, Conscientiousness, or Openness. The PDd group was significantly lower on Extroversion and scored significantly higher on Neuroticism than the PD and control groups. There were no significant differences on personality traits between the control group and the nondepressed PD group.
In order to investigate whether differences in disease features between PD and PDd influenced the differences in personality dimensions, we performed a MANCOVA, with disease severity, L-dopa use, and age at disease onset as covariates. The PDd group scored significantly higher on Neuroticism (F[1, 113]=14.644; p=0.000) and significantly lower on Extroversion (F[1, 113]=8.439; p=0.000), as compared with the PD group; there were no significant differences on Openness (F[1, 113]=2.450; p=0.120), Agreeableness (F[1, 113]=0.132; p=0.717), or Conscientiousness (F[1, 113]=1.125; p=0.291).
DISCUSSION
The purpose of this study was to explore the parkinsonian personality and its relation to concomitant depression. We found no evidence of a specific personality profile in nondepressed PD patients. However, depressed PD patients displayed traits similar to the personality profiles reported in other studies, with reduced Extroversion and increased Neuroticism. The depressed PD patients were significantly older than the PD patients without depression and the control participants. Differences in personality profiles did, however, remain significant after controlling for age. Also, the nondepressed PD group received significantly larger dopamine-agonist doses, which has been associated with a slight antidepressant effect. Hence, it is possible that depressive symptomatology would have been reduced in this group as a result of this medical regimen. Furthermore, the PDd group experienced significantly more action tremor, wearing-off, and postural instability. It is possible that more pronounced motor symptoms in this group could explain the lower Extroversion scores simply because of limitations imposed by the disease on physical mobility and social interactions. However, the results remained significant after controlling for disease severity. The increased tendency to wearing-off episodes in the PDd group may also be associated with an elevated propensity to experience fluctuations in affect, which could have an impact on the prevalence of depression in this group.
The study design does not allow for causal inferences between personality traits and depression in PD. Hence, it remains plausible that depression leads to changes in personality and vice versa. Furthermore, the co-occurrence of depression and high Neuroticism scores might be a reflection of the intertwined nature of traits (Neuroticism) and states (depression). In this regard, it may pose a particular challenge to distinguish Neuroticism from psychological state, since it displays some degree of correlation with several psychiatric symptoms,
25–27 and it has been suggested that a joint genetic factor underlies neuroticism and the risk of major depression.
28 Along this line, studies of healthy elderly subjects without a history of depression have identified neuroticism as an important predictor for late-life onset of depression.
12,29 Therefore, it is possible that increased neuroticism is manifest in a subgroup of PD patients premorbidly as a result of degeneration of dopaminergic neurons in the ventral tegmental area before the onset of depression. This subgroup could, in turn, subsequently be more susceptible to developing depression. Further studies are needed to ascertain such a relationship.
Interestingly, the present study failed to demonstrate differences in personality profiles between healthy elderly and nondepressed PD patients. Hence, unlike previous research, this study did not find evidence of a distinct parkinsonian personality applicable to PD, per se. One possible explanation for this finding is our use of the NEO-PI–R Short Version, whereas most of the recent research has used the TPQ (Tridimensional Personality Questionnaire) as a measure of personality traits.
8,9 The TPQ assesses three types of temperament: Novelty-Seeking, Reward-Dependence, and Harm-Avoidance. The traits are hypothesized to be expressions of particular neurotransmitters; that is, novelty-seeking is dependent on dopamine, harm-avoidance on serotonin, and reward-dependence on norepinephrine. However, disruptions of these three pathways are also believed to be implicated in PD depression.
30 Several studies have reported PD patients to be less novelty-seeking
31 and more harm-avoidant,
11,32,33 whereas others have only reported increased harm-avoidance.
10 Interestingly, harm-avoidance is positively correlated with the severity of depression in PD as well as in non-parkinsonian subjects.
33,34 The Harm-Avoidance trait on the TPQ is also positively correlated with Neuroticism on the NEO-PI–R and negatively correlated with Extroversion.
35 In the same regard, TPQ Novelty-Seeking is positively correlated with NEO-PI–R Extroversion.
35 Hence, it is interesting that studies using the TPQ found patients to differ on traits that were similar to the ones found in the present study of depressed PD patients. In relation to this research, the TPQ may face the same challenge of untangling contributions of PD and depression on personality profiles, because harm-avoidance appears to be especially sensitive to concomitant depression.
36,37 Furthermore, even though novelty-seeking is generally not found to correlate with depression in PD, direct comparison of depressed and nondepressed PD patients may yield different results. This was in part addressed by Menza and Mark,
14 who compared depressed and nondepressed PD patients by use of the TPQ. The authors reported a significant group difference on Harm-Avoidance only, and not on Novelty-Seeking. However, their patient group was subdivided into a depressed and a nondepressed group on the basis of the Zung Self-Rating Depression Scale (using a cutoff score of 48, being 1 SD from the mean), which has since been criticized for containing too many somatic items that overlap with PD symptomatology, thereby risking inflated depression prevalence.
38 Furthermore, no cutoff score for PD patients has been established on this scale, but, in medical settings, a cutoff score of ≥60 is recommended
39 this is higher than the cutoff score adopted by Menza and Mark.
14 Hence, there is a risk that depression rates were overestimated, which could have counterbalanced any potential difference in novelty-seeking between the two groups. Furthermore, the two PD groups were not compared with a control group. Further studies comparing depressed and nondepressed PD patients on the TPQ, using a standardized and validated measure of depression in PD, would be warranted.
Some limitations of the current study must be stressed. The study did not address the presence of apathy, which exists independently of depression in an estimated 12%–28.8% of PD patients.
40,41 However, applying the TPQ, Pluck and Brown
42 found no significant differences in personality measures between PD patients with high and low apathy scores. Unfortunately, the impact of apathy on NEO-PI–R has, to our knowledge, only been addressed in a study of college students, where students with high apathy scores were less agreeable, more introverted, and less conscientious.
43 If such a constellation of personality traits were applicable to a sizable nondepressed apathetic subgroup in our study, we would have expected to find significant differences in these traits between the PD and control group. This however, was not the case. On the other hand, the existence of apathy co-occurring with depression in the PDd group may have magnified our findings, as, for instance, reduced Extroversion scores could have been due to apathy. Second, the study did not use measures of cognitive deficits or dementia other than the MMSE, which was not available for all participants. Hence, it remains possible that some patients, for whom MMSE scores were unavailable, had dementia when they participated in the study. However, the medical charts of the patients in question were reviewed again, and none were diagnosed with dementia. Lastly, to our knowledge, none of the personality questionnaires (the NEO-PI–R, TPQ, or Karolinska Scales of Personality) commonly used to assess personality in PD has been validated specifically in this population. Such validation would be very important to estimate whether the content of the personality measures are related to functional, cognitive, or behavioral disability caused by PD.