Schizophrenia and obsessive compulsive disorder (OCD) are major mental disorders that often have their onset in adolescence, follow an episodic or chronic course, and result in considerable distress and disability.
1 Studies conducted over the past three decades estimate that 7.8% to 46% of people with schizophrenia have concomitant obsessive-compulsive (OC) symptoms
2–5; considerably higher than the rates of 1.2% to 2.4% reported in the general population.
6 This could suggest a pathophysiologic link between the two disorders.
OC symptoms may precede (predate) the onset of schizophrenia, may occur in the prodromal phase, or during the course of the illness. People with schizophrenia and comorbid obsessive compulsive symptoms are generally characterized by lower levels of social functioning, longer duration of hospitalization, more neurocognitive impairment, greater severity of negative symptoms, and greater propensity to develop extrapyramidal effects and treatment-resistance than those without OC symptoms.
2–5,7–11 Hence, OC symptoms may constitute a distinct cluster separate from psychosis in schizophrenia, thus raising the possibility of a distinct subtype of schizophrenia.
Many symptoms of schizophrenia can be explained by deficits of working memory that are, in turn, caused by dysfunction of dorsolateral prefrontal cortex (PFC).
12 The orbitofrontal cortex is a major neuroanatomical area associated with OCD;
13 hence, abnormalities in both dorsolateral PFC and orbitofrontal cortex may be involved in people with schizophrenia with comorbid OC symptoms.
Neurological soft signs and negative symptoms in schizophrenia patients may be considered as predisposing factors, as postulated by neurodevelopmental theory. It is speculated that schizophrenic patients with OC symptoms may have a greater propensity to basal ganglia dysfunction than those with schizophrenia-alone, which could result in increased parkinsonian symptoms.
14,15 Neuroimaging studies have revealed many common as well as dissimilar areas of structural changes in the brains of people with schizophrenia with and without OCD.
16–20 These common abnormalities, detected even early in the course of the disorder, and the overlap in the clinical presentations of these conditions, suggest a common neurodevelopmental origin for these conditions. They provide the basis for the suggestion that people with schizophrenia and comorbid OCD may be a distinct subtype of schizophrenia that some have called “schizo-obsessive disorder”
17,21 or “obsessive-compulsive schizophrenia”,
8 with further subgroups defined by time of onset of OCD in the course of the disorder and by other clinical or biological parameters.
22This study aimed to compare the pattern of soft neurological signs in drug-free patients with schizophrenia with and without OC symptoms, with a view to elucidating differences in the two subgroups that would point to a neurological substrate for the differing clinical presentations.
METHOD
This study was conducted over 18 months (July 1, 2005 to December 30, 2006) in the outpatient services of the Department of Psychiatry, Christian Medical College, a secondary and tertiary care, teaching, multispecialty medical institution in Vellore, Tamil Nadu, India. The protocol of this study was approved by the institutional review board (research and ethics committees).
Participants
Consecutively-registered outpatients over 18 years of age and diagnosed with schizophrenia (ICD-10) by clinicians were screened for inclusion in the study after we obtained informed consent.
Included were those with a diagnosis of schizophrenia, confirmed by using the International Classification of Diseases (ICD-10) Research Diagnostic Criteria,
23 and who were drug-naive or had not used antipsychotic or antidepressant drugs over the previous 3 months. Excluded were with those with a history of neurological illness, treatment with antipsychotics/antidepressants within the previous 3 months, seizure disorder, head injury, mental retardation, and substance abuse (except tobacco use).
Forty-four men and 21 women, with a mean age of 32.4 years (standard deviation [SD]: 9.8) met these criteria. We assessed each participant for the presence of neurological soft signs using the Neurological Evaluation Scale (NES), a structured scale presenting scores of subscales of motor coordination, sensory integration, sequencing of complex motor acts, and “other” signs.
14,15,24 The NES has 26 items rated on a scale of 0 to 2 (0: normal; 1: some disruption; 2: major disruption), according to standardized instructions. The Motor Coordination subscale combines the scores for tandem walk, rapid alternation movements, finger/thumb opposition, and the finger-to-nose test. This subscale assesses fronto-cerebellar functions. The Sensory Integration subscale includes audiovisual integration, stereognosis, graphesthesia, extinction, and right/left confusion. These tests evaluate parietal lobe functions. Sequencing of Motor Acts includes the first-ring test, the first-edge-palm test, the Ozeretski test, and rhythm-taping test. This subscale assesses prefrontal functions. The other subscales are the Primitive Reflex subscale that assesses frontal lobe functions and the Hard Neurological Signs subscale that evaluates the CNS, including cranial nerves.
15Classification of participants as Cases (those with OC symptoms) and Controls (those without OC symptoms) was done using the checklist of the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)
25 after NES assessments were completed. Participants were also rated for severity on the Y-BOCS. Sociodemographic details, family history, and clinical data were also obtained from interviews with participants and their relatives and from case records. Each participant was also rated using the Positive and Negative Symptom Scale (PANSS),
26 and the Clinical Global Impression—Severity scale (CGI).
27Data Analysis
We compared categorical data between Cases and Controls with the chi-square test or Fisher's exact test, and continuous variables with the t-test or Mann-Whitney U test (if data were skewed). Pearson's correlations between scale scores on NES and the PANSS scores as well as NES total subscale scores and Y-BOCS total and subscale scores were computed after testing the data for normality and linearity of distributions. All tests were two-tailed, with a significance level of 0.05. All comparative analyses were conducted using the Statistical Package for Social Sciences, Version 10. (SPSS, Inc.).
RESULTS
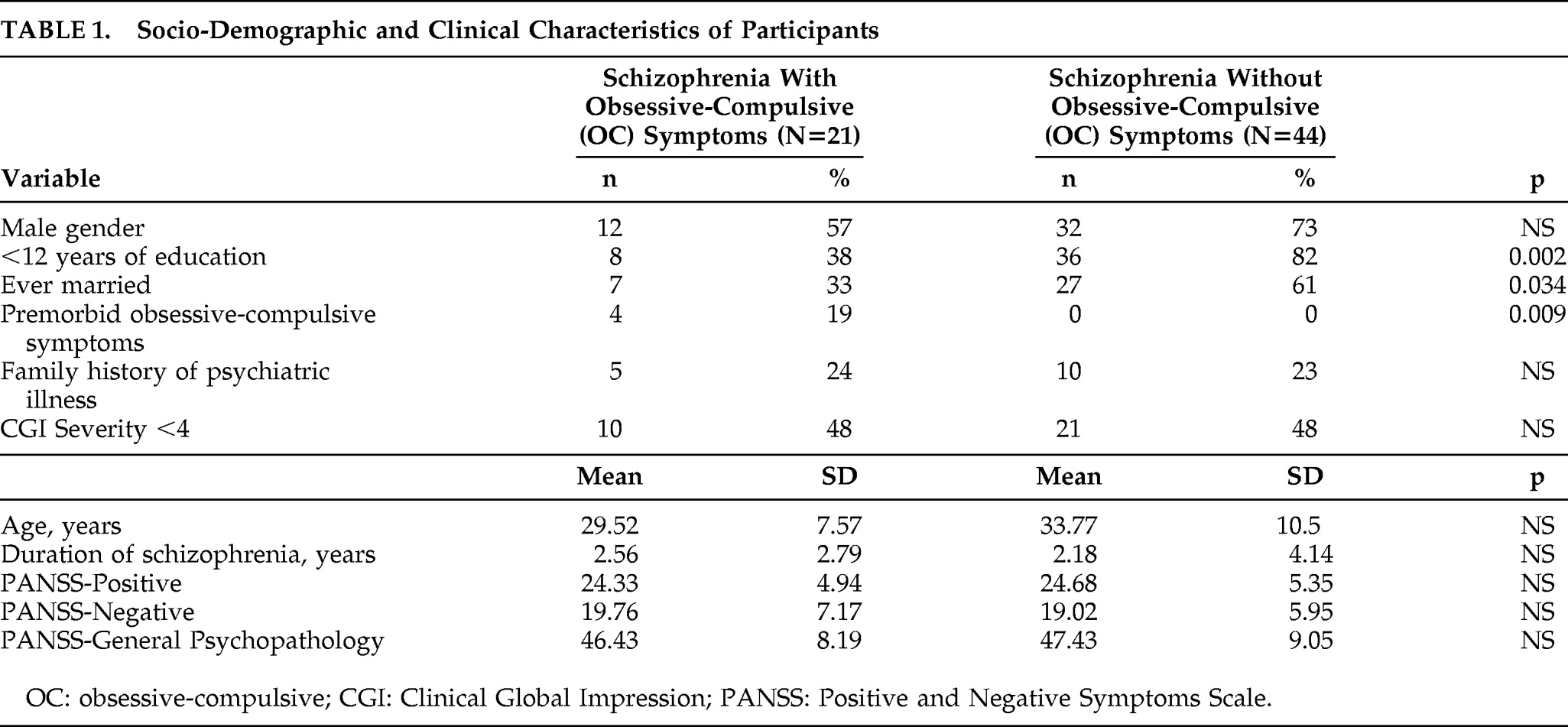
Demographic and clinical characteristics of the study participants are shown in
Table 1. There was no significant difference in the proportion of men and women or the mean age of the study participants in the two groups. The participants with schizophrenia and OC symptoms had significantly more years of education than those without and were also more likely to be single. They also differed in proportions with premorbid OC symptoms, but were similar in other demographic and clinical characteristics. Scores on the PANSS and for Clinical Global Impression Severity scale did not differ between those with and without OC symptoms.
Participants without OC symptoms did not score on the Y-BOCS, whereas the mean scores for those with OC symptoms were 19.4 (SD=5.6) for the Total score, 9.0 (SD=4.2) on the Obsessions subscale and 10.4 (SD=3.2) on the Compulsion subscale. Of these 21 people, 5 were rated mild for severity on the Y-BOCS, 15 as moderate, and 1 rated as severe; 14 had multiple symptoms, and the others had single symptoms related to contamination, washing, symmetry, hoarding, or compulsions to utter or ask.
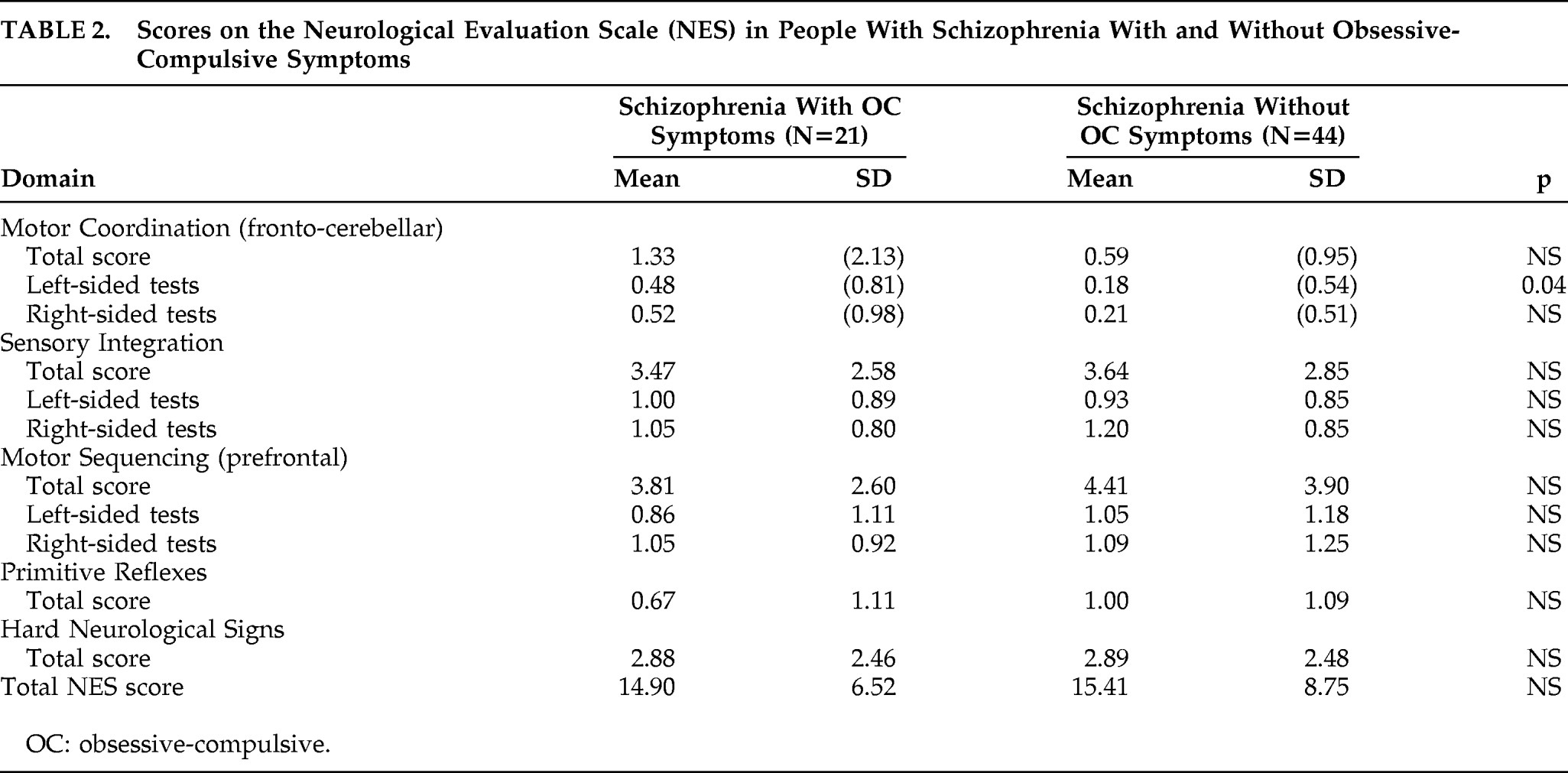
People with schizophrenia with and without OC symptoms did not differ significantly in total scores on the NES or in any of the individual tests (
Table 2). However, the two groups differed significantly in left-sided tests on the Motor Coordination subscale of the NES, which assesses fronto-cerebellar functions, although this was not observed in right-sided tests (
Table 2). All people with OC symptoms were assessed to have left cerebral dominance as evaluated by NES tests for handedness and footedness, whereas 40 of those without OC symptoms (91%) showed left cerebral dominance on these tests.
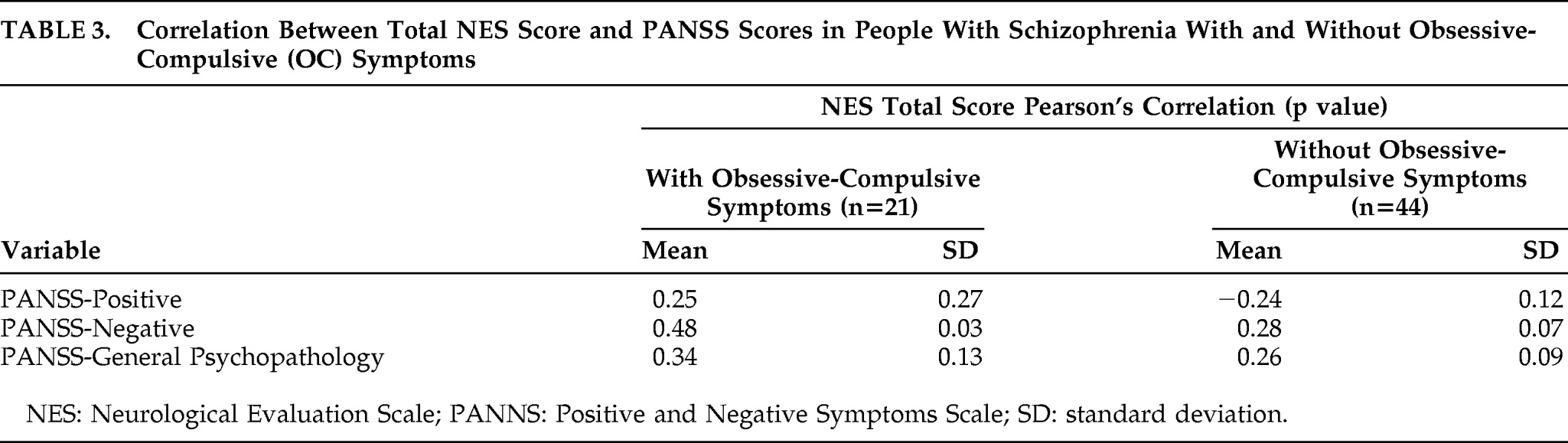
There was a significant positive correlation found between the PANSS-Negative symptoms scores and the NES total scores in the subgroup with OC symptoms, but not those without OC symptoms (
Table 3).
Scores on the NES (total or subscales) did not correlate significantly with Y-BOCS total score or subscale scores for obsessions or compulsions, nor were significant correlations observed between Y-BOCS total or subscale scores and PANSS scores for Positive, Negative or General psychopathology scales (data available on request).
DISCUSSION
This cross-sectional study revealed many similarities and some differences in people with schizophrenia with and without OC symptoms. The two groups did not differ at baseline in clinical measures of schizophrenia psychopathology. Those with OC symptoms were better educated and more likely to have been ever married, although there were no differences in their socioeconomic status or duration or severity of the symptoms of schizophrenia. In the group with OC symptoms, 19% reported that OC symptoms antedated the development of the symptoms of schizophrenia, and, since all were antipsychotic drug-naive, the role of drugs in the development of OC symptoms can be ruled out in this sample.
The two groups did not differ significantly in total NES scores, but did differ significantly in performance in the aggregation of tests that form the motor coordination subscale of the NES that assesses fronto-cerebellar functions. This difference was seen only for the tests assessing left-sided fronto-cerebellar functions. This differential performance suggests that people with schizophrenia and OC symptoms differ from those without OC symptoms in fronto-cerebellar circuitry involving the left side.
We had originally hoped to recruit a larger number of people with schizophrenia and OC symptoms, and it is possible that the absence of significant difference in NES total scores or for subtest scores may be due to the relatively small sample (Type II error). However, even a trend toward significant differences was not seen in these scores, so this does not seem the most likely explanation for the findings.
The other possibility that needs consideration in explaining the difference in NES scores of motor coordination of left-sided tests is a type I error or a false-positive finding due to improper methods of assessment. Studies assessing neurological soft signs in people with schizophrenia should take cognizance of the direct and indirect effects of antipsychotic drugs on such evaluations. Motor symptoms due to antipsychotic drugs such as tremor, adventitious overflow, rigidity, and poor balance may be erroneously rated as neurological signs. Higher antipsychotic doses can result in poorer performance for prefrontal signs and better performance in parietal and non-localizing signs.
28 Antipsychotic-treated patients are reported to evidence a higher rate of soft neurological signs than antipsychotic-naive people in structured assessments.
29 In this study, we included participants who were drug-naive or free of antipsychotic or antidepressant drugs for 3 months in order to eliminate the effects these drugs may have on clinical symptoms or on test performance.
It is possible, however, that the significant difference in motor coordination scores for left-sided tests observed between those with and without OC symptoms is a chance association due to the multiplicity of statistical tests performed, and correcting for this would render the observed statistical difference nonsignificant. Hence, these results need replication in larger samples, although it would be imprudent to ignore the observed differences altogether.
Although there is no published research investigating the unique neurotransmitter involvement in people with schizophrenia and obsessive-compulsive symptoms, serotonin and dopamine have most consistently emerged as the principal neurotransmitters involved in both disorders. In schizophrenia, the dopamine hypothesis has been considered as the fundamental neurochemical model, and this is most strongly supported by successful treatment of the disorder with dopamine-receptor antagonists.
30 However, the efficacy of the serotonin—dopamine receptor antagonists in the treatment of schizophrenia additionally supports the importance of the serotonergic system in the pathophysiology of this disorder and may reflect the modulation of dopaminergic pathways by serotonin receptors.
31 In OCD, the serotonin hypothesis is well established and is supported by successful treatment of the disorder with serotonin-reuptake inhibitors.
32 Since only 40%–60% of patients with OCD exhibit response to monotherapy with selective serotonin-reuptake inhibitors, other neurotransmitter systems may be involved in the pathophysiology of OCD.
32 Evidence suggesting the role of dopaminergic system in the pathophysiology of OCD includes the role of dopamine in stereotypic behaviors in animal models, the etiologic role of dopamine in Tourette's disorder, and successful treatment of refractory OCD with dopamine-receptor antagonists.
33–35 It is, therefore, reasonable to presume that people with schizophrenia with OC symptoms have abnormalities in both serotonin and dopaminergic systems and hence require drugs that affect both neurotransmitter systems. However, this requires confirmation in large, randomized trials.
Schizophrenia and OCD are both considered as disorders of cortico-striato-thalamo-cortical circuitry,
36,37 but the cortical areas involved in these disorders are different. In schizophrenia, the main cortical area involved is the dorsolateral prefrontal cortex, and, in OCD, it is the orbitofrontal cortex.
36,37 The NES scale does not specifically assess orbitofrontal cortex functions, and this could explain the lack of significant differences in many of the NES items or the total score. This leaves us with the possibility that the significant difference between the two groups in the motor coordination subscale on the left side, if replicated in larger samples or evaluated via other investigative approaches, is due to differences in fronto-cerebellar circuitry in patients with schizophrenia with obsessive-compulsive symptoms, with possible neurodevelopmental origins.
Acknowledgments
We thank all participants for their time and cooperation with the study. This study was funded by the Fluid Research Fund of the Christian Medical College, Vellore. The authors declare no competing interests.