There has been considerable controversy about the impact of playing American professional football on long-term brain function.
1 At the end of 2009, the controversy was significantly fueled by a study sponsored by the National Football League (NFL), which found that retired players age 30–49 receive a dementia-related diagnosis at a rate of 1.9%, or 20 times the rate of age-matched populations, and 6.1% of players over the age of 50 receive a dementia-related diagnosis, representing five times the national average of 1.2%.
2To date, there have been no published functional brain-imaging studies on active or retired NFL players, even though brain injuries are common, and their incidence has been associated with mild cognitive impairment
3 and depression.
4Studying the brain function in a large group of living players is important to better understand whether there are widespread persistent negative brain effects from playing professional football and, if so, to evaluate the potential for rehabilitation. Both brain single photon emission computed tomography (SPECT) imaging
5 and quantitative EEG (qEEG) have substantial data for evaluating traumatic brain injury (TBI).
6 Brain SPECT has been shown to be more sensitive than standard CT or MRI
7,8 in evaluating TBI.
Our a priori hypothesis was that, relative to a matched healthy-comparison group, active and retired NFL players as a group would exhibit significant decreases in regional cerebral blood flow (rCBF) in the frontal, temporal, and occipital lobe regions of the brain, consistent with previous brain trauma, and this would result in compromised neuropsychological functioning.
METHOD
We recruited 100 active and retired NFL players, representing 27 teams and all positions (see
Table 1 for summary). Players were recruited from retired NFL Players Association meetings and by participants informing other players about the study. Each player met our inclusion criteria of being on an active NFL roster for a minimum of 3 years. We excluded any subjects who could not cease taking psychoactive medications (recreational or otherwise) for an appropriate washout period before scanning was done. All participants received an explanation of the research study and gave written informed consent in accordance with an institutional review board-approved protocol.
Each participant was interviewed by a physician and completed a detailed history, including a 315-question DSM-IV-driven questionnaire to assess overall general and mental health. Waist and height sizes were obtained on all participants, and waist-to-height ratios were calculated. Brain concussion and loss-of-consciousness data were acquired from each player. Subjects were asked to recall the number of concussions they had experienced throughout their lifetime, including those obtained while playing high school, college, and professional football. We used the Centers for Disease Control and Prevention (CDC) definition
9 of concussions: “conditions of temporarily altered mental status as a result of head trauma” that may or may not involve a loss of consciousness. Understanding that self-report data, especially from many years past, is potentially unreliable, players were also asked about periods where they experienced a distinct loss of consciousness. Past medical records were not available for this study, which is a limitation. Many subjects played in an era of football where concussions were not taken as seriously as they are today.
Three computerized neuropsychological tests were given to each player. These tests included the MicroCog Assessment of Cognitive Functioning,
10 which contains 12 subtests that represent functioning in five core neurocognitive domains, including attention/mental control, memory, reasoning, spatial processing and reaction time. The Microcog Assessment of Cognitive Functioning scores were compared with its own standardized sample (N=810), chosen to be representative to the U.S. population of adults between the ages of 18 and 89 with regard to education, gender, and ethnicity. Because our sample had a higher percentage of African Americans (33%) than the 13% in the U.S. population, further ethnic evaluations were performed on the MicroCog. Participants also took the Conners' Continuous Performance Test II,
11 which measures response inhibition and attention, and is a validated screening tool that assigns a clinical probability of having attention-deficit hyperactivity disorder (ADHD), based on its own large normative and clinical sample database, which did not show an effect of ethnicity.
12 Participants were also given the Mild Cognitive Impairment Screen,
13 a screening tool found to be reliable in distinguishing mild cognitive impairment from normal.
14 The effect of ethnicity on this test has been studied and found to be essentially zero.
15Each player underwent high-resolution brain SPECT imaging and qEEG. For SPECT, we used already-acquired right-handed men as a comparison group (N=20; mean age=50.0, range=27–83, SD=16.1). The ethnic makeup of the comparison group was Caucasian (80%), Hispanic (10%), African American (5%), and Asian (5%). The healthy-comparison subjects were screened using clinical interviews, the same 315-question DSM-IV-driven questionnaire used in this study, which was also filled out by a significant-other, a Structured Clinical Interview for DSM-IV (SCID), the Beck Depression Inventory, the Mild Cognitive Impairment Screen, and the MMPI. All scores were in the normal range. Healthy-comparison subjects also reported no brain injuries or head trauma upon being asked repeatedly in multiple ways. They were not taking any medications at the time of evaluation and had no medical illnesses. As part of the SPECT procedure, the healthy-comparison men also took a Conners' Continuous Performance test II. Only healthy comparison subjects who scored in the normal range on the Conners' test were used for this study. Of note, we did not have a sufficient number of African Americans in our Normal-Control Subject database for comparison with the NFL players. We added an additional analysis, comparing Caucasian players with Caucasian comparison subjects to compensate for this issue.
The qEEG data were compared against a nationally published normative database.
SPECT Acquisition and Analysis
We used SPECT to measure rCBF in both players and comparison subjects. Each participant received an age/weight-appropriate dose of technetium-99m hexamethylpropyleneamine oxime (HMPAO) intravenously. Participants were injected in normal lighting while they performed the Conners' Continuous Performance test II. The radiopharmaceutical was injected 3 minutes after starting the 15-minute test. All participants completed the task. The individuals were then scanned 30 minutes later using a high-resolution Picker Prism 3000 triple-headed gamma camera with fan beam collimators, acquiring data in 128×128 matrices, yielding 120 images per scan with each image separated by 3° spanning 360°.
SPECT data were processed and attenuation correction performed using general linear (Chang) methods. All images were reconstructed and resliced using an oblique reformatting program, according to anterior–posterior commissure line so final images were similarly aligned for analysis.
Differences in HMPAO uptake were analyzed using SPM8 software (Wellcome Department of Cognitive Neurology, London) implemented on the Matlab platform (MathWorks Inc., Sherborn, Mass., 2004). Statistical parametric maps are spatially extended statistical processes that are constructed to test hypotheses about regionally specific effects in neuroimaging data. Statistical parametric mapping combines the general linear model and the theory of Gaussian random fields to make statistical inferences about regional effects.
16 The images were spatially normalized using a 12-parameter affine transformation, followed by nonlinear deformations
17 to minimize the residual sum-of-squares between each scan, and a reference or template image conforming to the standard space defined by the Montreal Neurological Institute template. The original image matrix obtained at 128×128×29 with voxel sizes of 2.16 mm×2.16 mm×6.48 mm were transformed and resliced to a 79×95×68 matrix with voxel sizes of 2 mm×2 mm×2 mm, consistent with the Montreal Neurological Institute template. Images were smoothed using an 8-mm full width at half maximum isotropic Gaussian kernel. The two-sample
t-test design was used with analysis of covariance (ANCOVA) by subject regressors to account for differences in subject-specific regional response to changes in global cerebral blood flow (CBF), with age as a covariate. To test our hypotheses regarding regionally-specific condition effects, the estimates were compared using linear contrasts. The resulting set of voxel values for each contrast represents a parametric mapping of the
t-statistics, statistical parametric map (
t), which were transformed into the unit normal distribution statistical parametric map (z) and thresholded at
t=8.85; p<0.0001, corrected for multiple comparisons using the family-wise error-rate correction in SPM8.
qEEG Acquisition and Analysis
Quantitative EEG is the measurement of electrical patterns at the surface of the scalp, which reflect cortical electrical activity. All of our measurements were obtained with the subjects' eyes closed and were sampled at 200 Hz, using the international 10/20 system of electrode placement and manual and automatic artifact removal. Test–retest reliability was greater than 0.9 for all participants.
18 There are two categories of measurement using qEEG: the first category involves amplitude or power at different frequencies, including three-dimenional sources; the second category involves network measures, such as conduction velocities (phase differences), coupling magnitudes (coherence) and thalamo-cortical phase shifts and phase locks.
19–22 Previous qEEG studies have shown that network measures are the most sensitive in the evaluation of mild-to-severe TBI.
23,24 Comparison with an age-matched qEEG normative database was used for the three-dimensional source analyses, which were demographically balanced, with 24.2% African American clinically healthy subjects.
25,26 The effect of ethnicity for qEEG was studied and found to be essentially zero.
27 None of the healthy-comparison subjects exhibited focal brain abnormalities, and all of the healthy-comparison subjects were without a history of neurological disorders, including TBI.
RESULTS
See
Table 1 for demographic characteristics, handedness, positions played, reported number of loss of consciousness data, incidence of depression, and waist/height ratio. See
Table 2 for neuropsychological test results. The results of the MicroCog Assessment of Cognitive Functioning revealed that players scored in the bottom half of the percentile placements on all measures except spatial processing and reaction-time, which were both in the top half of the percentile placements. This was true for both Caucasian and African American players, although African Americans scored lower on all measures, as a group.
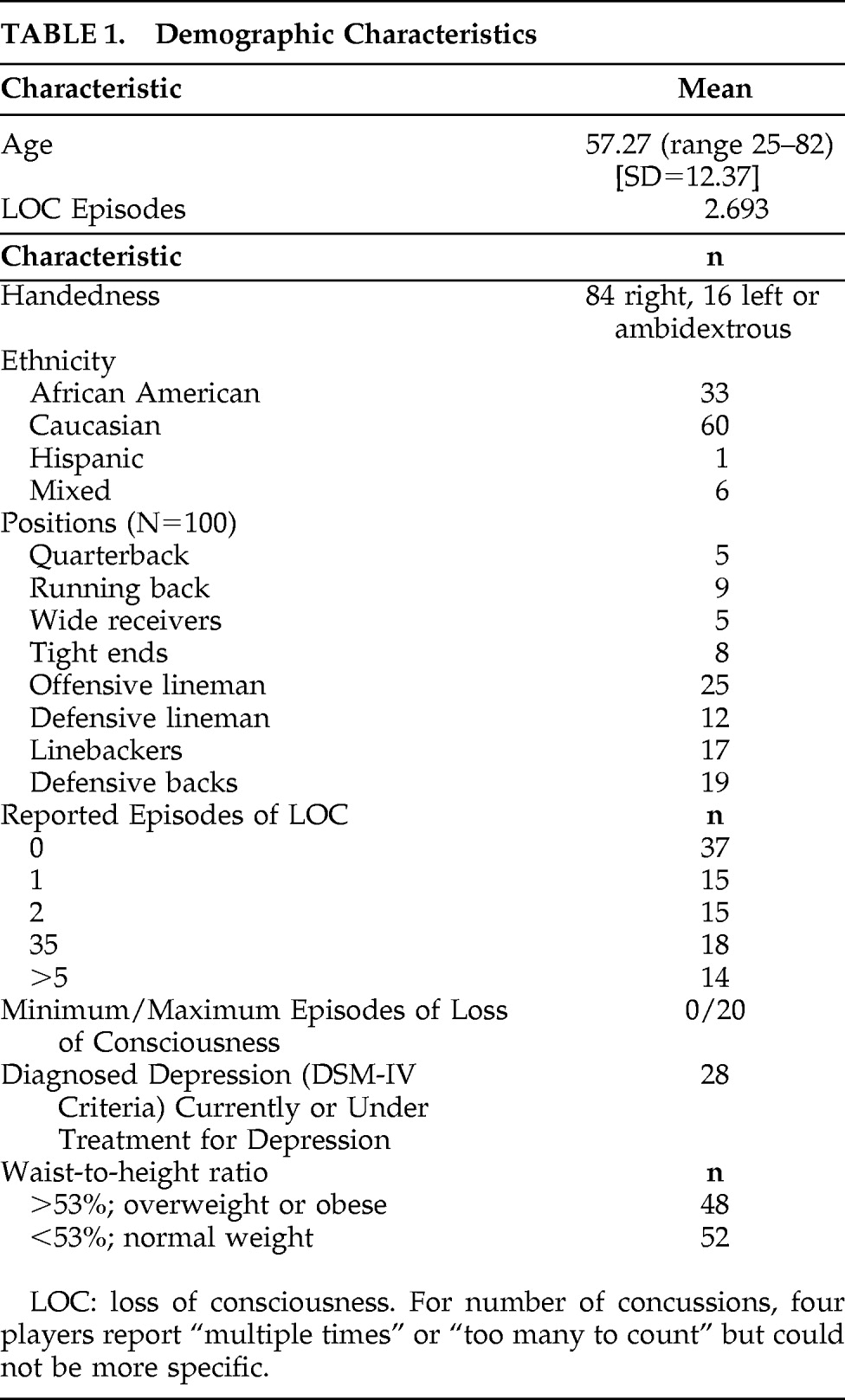
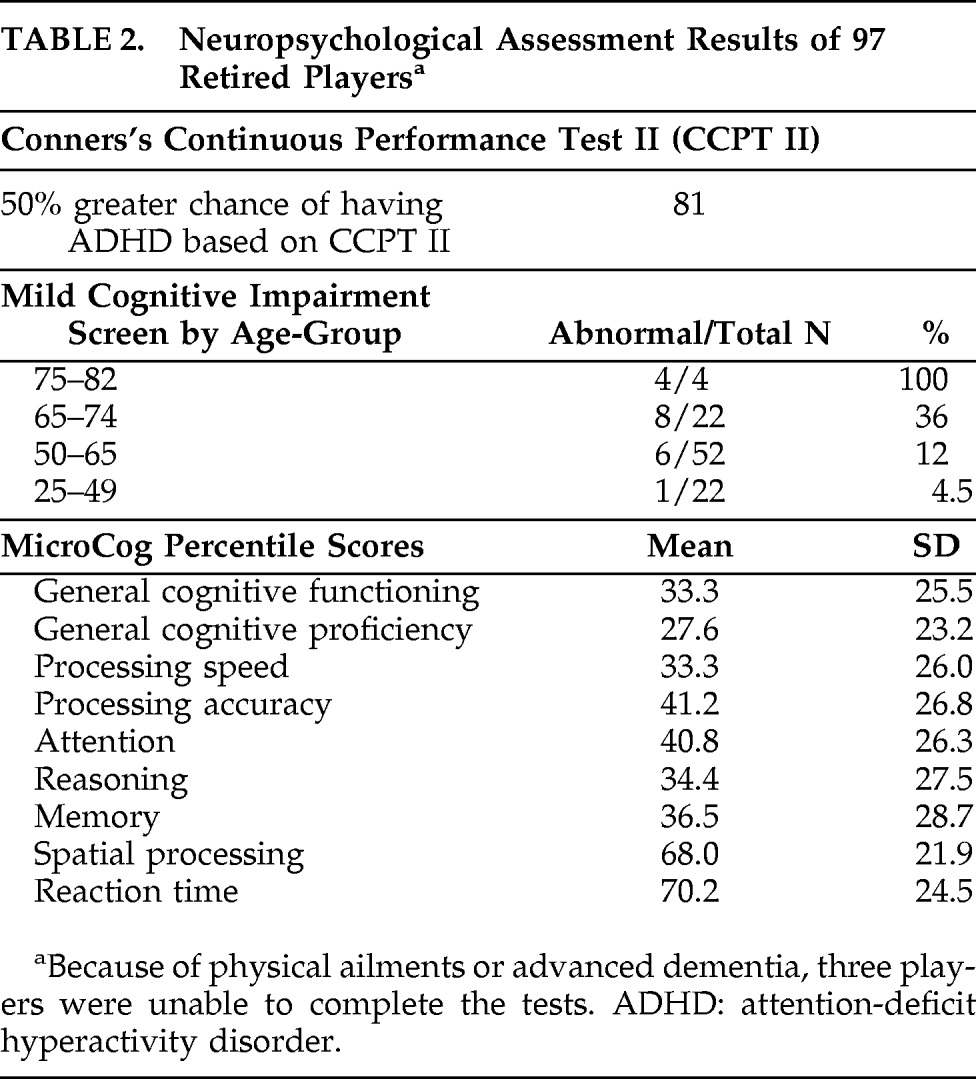
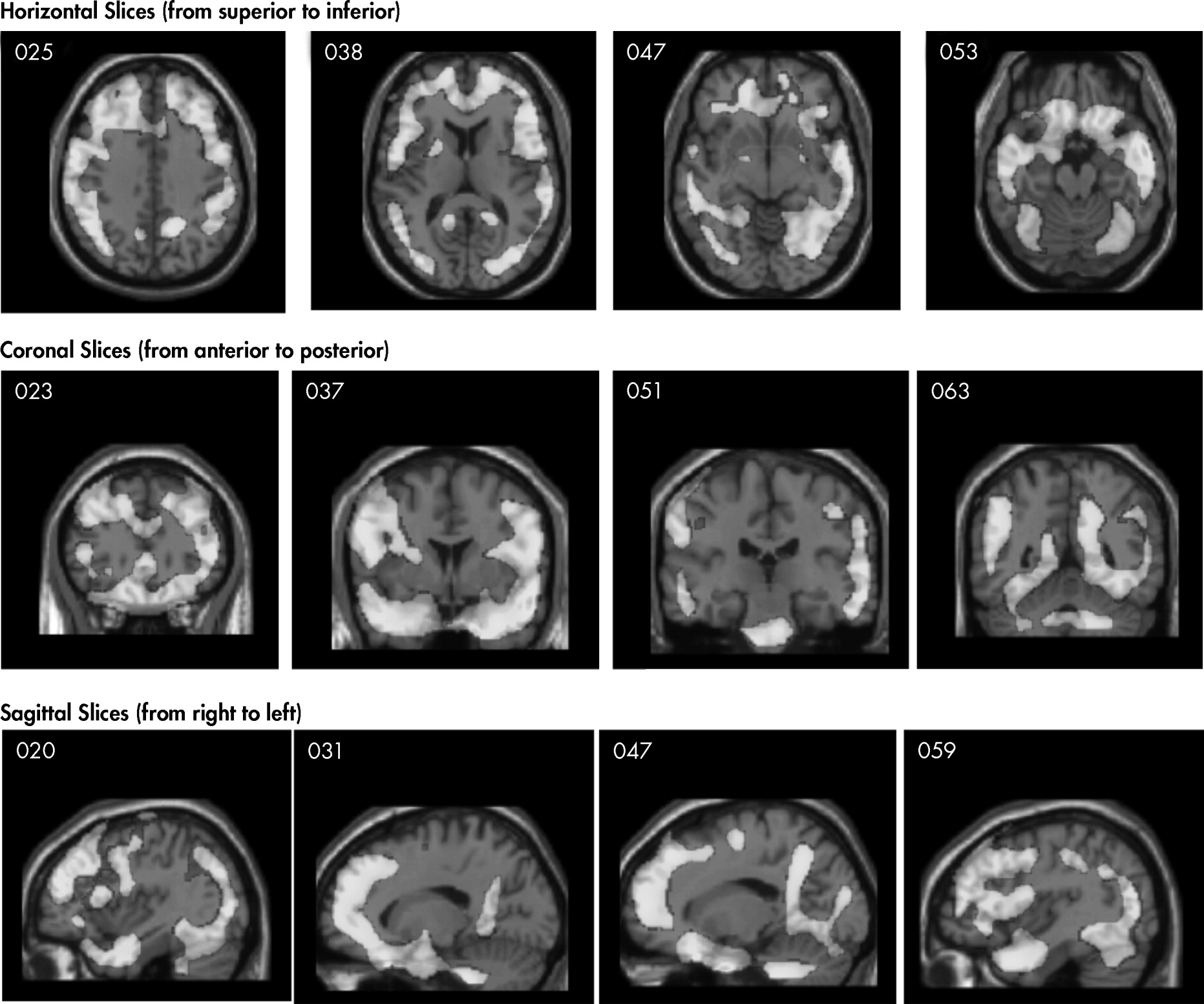
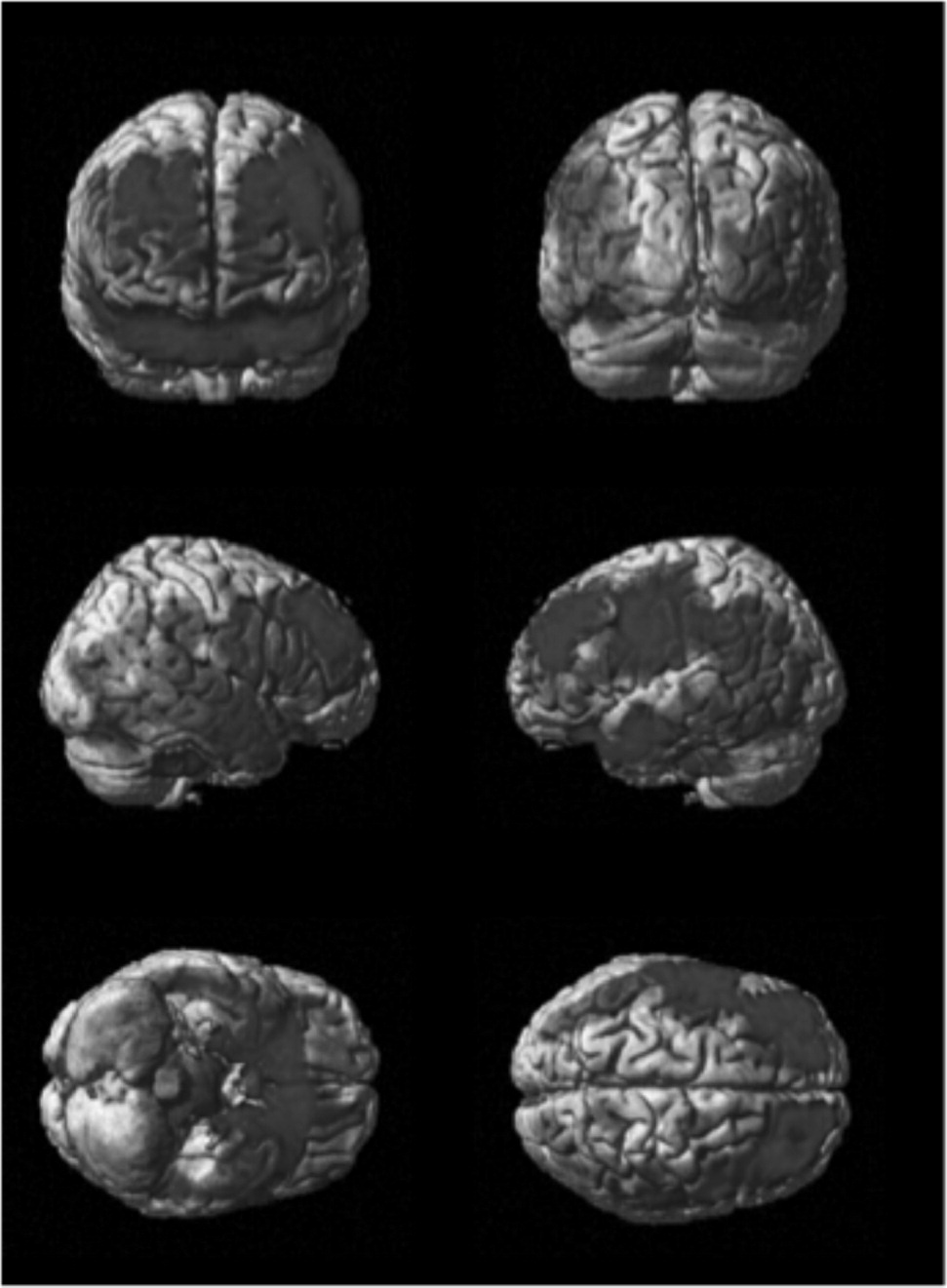
Figure 1 and
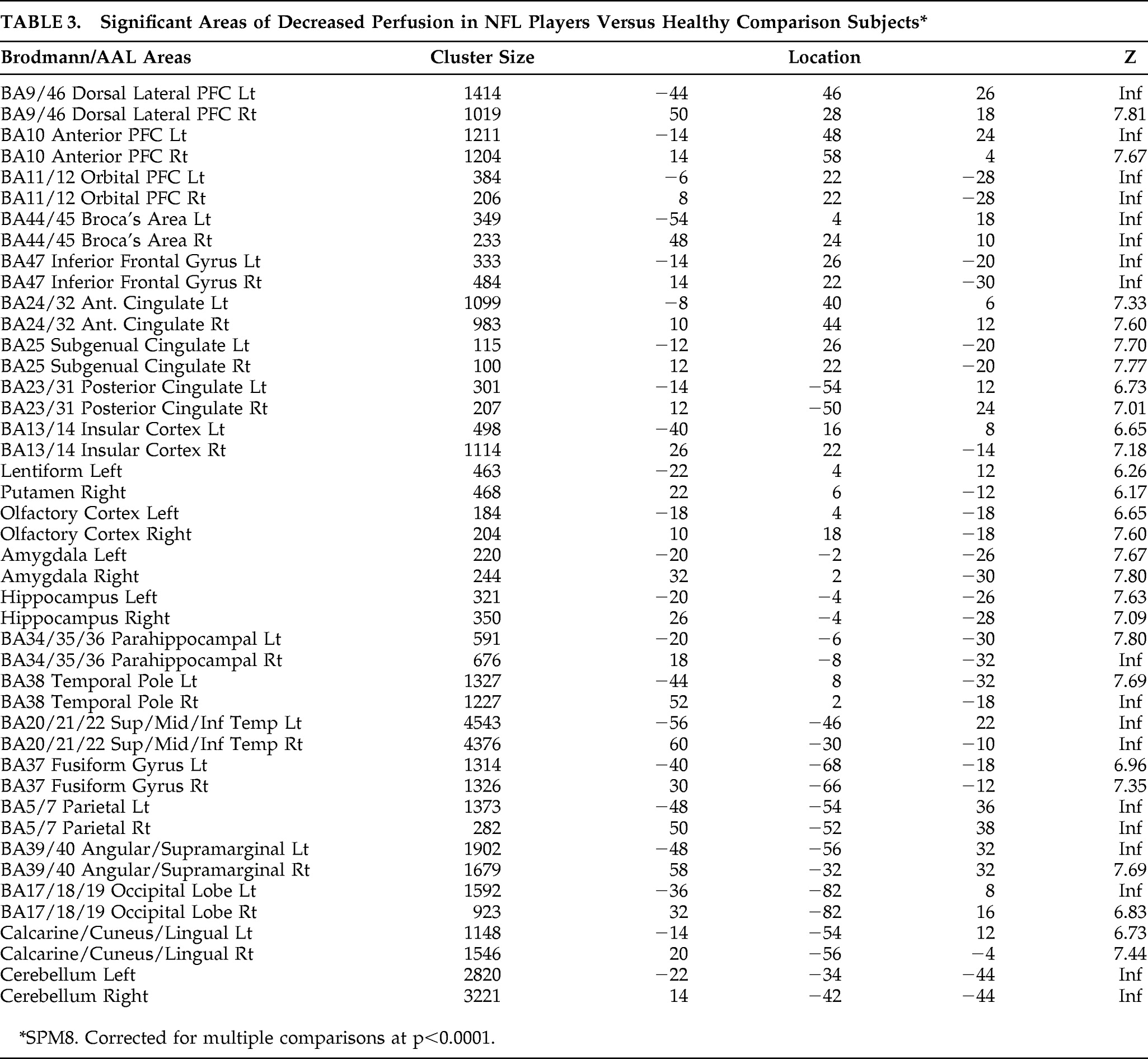
Figure 2 show differences between NFL players and the healthy-comparison subjects on SPECT, using age as a nuisance covariate. No areas of increased perfusion were seen. There were global decreases across the whole brain, especially in the prefrontal, temporal, parietal, and occipital lobes, anterior and posterior cingulate gyrus, and cerebellum. The decreases were significant at p<0.0001 (family-wise error corrected for multiple comparisons). The Brodmann's areas that showed these decreases are listed in
Table 3. When we felt Brodmann's areas were insufficient, we also used areas defined by the Automated Anatomical Labeling Atlas.
Because African Americans were more numerous in our sample than in the general population, and also because our healthy-comparison group did not have an adequate number of African American men, we performed another analysis using only Caucasian players (n=60) versus Caucasian comparison subjects.
16 The results were essentially the same, showing global decreases at p<0.001, family-wise error, and no increases.
We also performed two additional analyses on our data. First, we examined players who reported a high number of loss-of-consciousness episodes (three or more) versus players who reported no loss-of-consciousness episodes. The total group size was 32 with high loss-of-consciousness and 32 with no loss-of-consciousness. The analysis yielded no significant differences with family-wise error, multiple-comparison corrections. Without multiple comparisons corrections, at p<0.01, there were several areas of increased activity in the lateral prefrontal cortices and areas of decreased perfusion in the temporal, parietal, and occipital lobes in the high loss-of-consciousness group. In the second additional analysis, we examined players who played a high number of professional games (≥200) versus those who played a relatively low number of professional games (≤50). Twenty-six players in our study played 50 or fewer games, and 10 players played 200 or more games. Again, no significant differences survived family-wise error corrections for multiple comparisons, but there were significant decreases in the 200+-games group compared to the 50− group, without corrections, for multiple comparisons at p<0.001 in the prefrontal cortex, temporal, parietal, and occipital lobes and in the cerebellum.
The qEEG findings were consistent with the SPECT findings, and showed significantly elevated slow waves in the bilateral temporal regions and bilateral frontal lobes, and reduced power at the higher frequencies. Because of the brain–bone interface, focal deviations from healthy-comparison subjects in the frontal and temporal lobes are a common qEEG finding in TBI patients.
28,29 Also, changes in the conduction velocities and network synchrony between different brain regions were deviant from normal relative to a group of healthy-comparison subjects. A more detailed analysis of qEEG connectivity measures is in preparation for a future publication.
CONCLUSION
Omalu et al.
30,31 and McKee et al.
32 have reported finding excess tau protein deposits at autopsy (called chronic traumatic encephalopathy or CTE) in a number of deceased retired professional football players. Ours is the first study using multiple brain-imaging methods and neuropsychological testing to demonstrate significant brain abnormalities in a large group of living active and retired professional football players.
On SPECT, significant decreases in regional cerebral blood flow were seen across the whole brain, especially in the prefrontal poles, temporal poles, occipital lobes, anterior cingulate gyrus, and cerebellum. This pattern is consistent with the lasting effects of TBI.
33 We also found significant decreases in the posterior cingulate gyrus and hippocampus, areas implicated in dementia.
34The Mild Cognitive Impairment Screen has been found to be a reliable tool in distinguishing mild cognitive impairment and dementia from normal, which is an important issue in this population. In the general population, the prevalence of mild cognitive impairment or dementia under age 50 is typically small: 0.1%.
35 In our sample, 4.5% of subjects in this age range scored in the abnormal range on the Screen. The general incidence of dementia between age 50 to 65 ranges from 0.3% to 2.2%; in our sample 12% in this age-group had abnormal Screen studies. The general incidence of dementia at age 65 is 2.2%, increasing to 6.5% at age 75. In our sample, 36% of players in this age range had an abnormal Screen study. Over age 74, 100% of players had abnormal Screen scores.
On the Conners' Continuous Performance Test II, 80% of the NFL group scored 50% or greater as having “probable” ADHD. Imaging studies have shown that an extensive brain network is activated during this task, including frontal, temporal, and occipital areas;
36 all of these areas showed significant decreases on the SPECT scans. Some players did have childhood histories consistent with ADHD, but at a much lower level.
Depression is associated with lower brain perfusion, especially in the prefrontal cortices, and the group of NFL players showed a significantly higher incidence of depression (28%) than is found in the general population (9.5%).
37 Depression has also been associated with brain injury.
38 Also, we found decreases in Brodmann's area 25, which has been reported in resistant depression
39 and suicide.
40 The qEEG findings showed focal frontal and temporal lobe deviations involving brain regions that are vulnerable to rapid acceleration/deceleration injuries.
41,42Other factors may be involved in these findings, including past drug or alcohol abuse, depression, steroid abuse, and brain injuries outside of the NFL, such as from high school or college sports or motor vehicle accidents. Also, a recent study showed that being overweight or obese is associated with smaller brain volume.
43 Forty-eight percent of players had a waist-to-height ratio over 53%, which is associated with increased cardiovascular disease risk factors,
44 as compared with 33% in the general adult population.
45It is also known that African-Americans in the United States suffer disproportionally from disorders such as hypertension and diabetes, which are known to increase risk for cardiovascular problems, including strokes and vascular dementia. Since we had a high African American population among our study subjects and not an equivalent percentage of healthy-comparison subjects, we performed an additional SPECT analysis comparing Caucasian players with Caucasian healthy subjects and found the same basic pattern seen for the total group comparisons.
Also, our analysis comparing high versus low loss-of-consciousness groups did not reveal further brain decreases when subjected to multiple comparisons corrections as hypothesized. One possible explanation is that the decreased perfusion is a result of the repetitive blows to the head, rather than single-episode traumas. Also, the lack of additional results in our comparison of high versus low number of games played suggests that by the time players enter into professional football, where they have usually already played for 8 years or more at a high level, they may have already experienced some problems in function. Screening players upon entering the NFL may be an important step to understanding this problem further.
There are a number of limitations to our study. The sample may not be representative of all players, because subjects needed to be able to travel to the study location and have social contact that allowed them to learn about and participate in the study. Players who were homeless, had dementia, or were of low income may have had a more difficult time participating. Also, it is possible that players who were more concerned about their memory or mood issues were more inclined to volunteer. Also, we were unable to obtain the complete medical records on many of our participants. The information on concussions and loss of consciousness was based on player or family recall, which may be subject to error.
The results of this study suggest that playing professional football is associated with a significantly higher risk for permanent brain damage. Further imaging studies, particularly longitudinal in nature, and neuropsychological assessments using larger, randomly selected groups, with ethnicity-matched normal subjects is warranted, as well as studies to evaluate prevention and rehabilitation strategies.
Acknowledgments
The authors thank Anthony Davis, Marvin Smith, Reggie Berry, Dave Pear, Robert Lee and all the retired players for their assistance. Also, we are grateful to Christine Kraus, Ph.D., Steve Stockdale, Ph.D., William Shankle, M.D., and Manuel Trujillo, M.D., for their consultation. This study was approved and supervised by an institutional review board.
Players were recruited with the help of the Los Angeles Chapter of the Retired NFL Players Association, The Summit, and Dave Pear's Blog. No author reports a conflict of interest or financial disclosure.