Mild traumatic brain injury (mTBI) is a diagnostic challenge because most patients have absent or minimal duration of loss of consciousness, no obvious neurological deficits, and normal brain scans when assessed by conventional neuroimaging methods such as computerized tomography (CT) or magnetic resonance imaging (MRI). In addition to the absence of diagnostic tests to diagnose mTBI, there are no methods that can accurately predict which patients are at risk for developing persistent problems. Normal sleep processes involve a coordinated, orchestrated, synchronous process involving structures in the brainstem, midbrain, and cortex.
1 In the current study, we tested the hypothesis that mTBI is associated with a disruption in normal sleep processes via diffuse axonal shearing and subsequent metabolic abnormalities associated with neuronal injury.
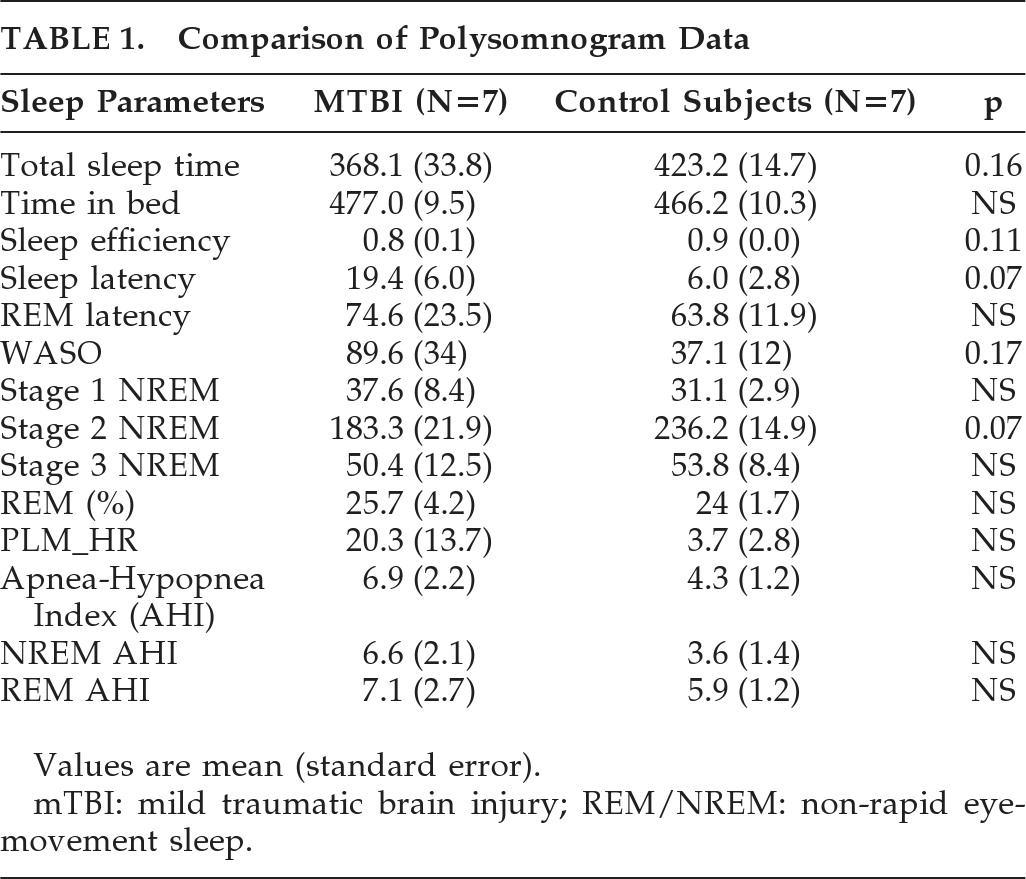
To test our hypothesis, we conducted a pilot exploratory study to characterize macro- and microarchitecture of sleep soon after mTBI. The primary goal of the study was to compare sleep polysomnograms (PSG) and sleep EEG power spectra in patients with acute mTBI (<1 week after injury) with age- and race-matched healthy historical control subjects.
METHOD
Subject Recruitment
mTBI patients were recruited from the community via advertisements. Inclusion criteria for study participation were the following: 1) history of closed head injury, defined as externally-inflicted trauma without skull fracture; 2) Glasgow Coma Scale (GCS) of ≥13; 3) American Congress of Rehabilitation Medicine (ACRM) diagnostic criteria of mild TBI fulfilled; 4) time duration since injury ≤1 week; 5) sufficient cognitive capacity to provide informed consent; 6) age 18–45 years; and 7) willingness to have two overnight polysomnograms and stay at the research sleep lab for the day between the two studies. Exclusion criteria included 1) history of stroke, encephalitis, seizures, or any other pre-TBI neurological diseases; 2) history of mental retardation; 3) history of open head injury (penetration of dura mater); 4) current or within-the-last-month use of psychotropics, including benzodiazepines, hypnotics, anxiolytics, opiates, stimulants, antidepressants, antipsychotics, or mood stabilizers; 6) history of alcohol or substance abuse/dependence; 7) blood alcohol level >100 mg/dl; 8) being a prisoner; 9) presence of pre-TBI sleep problems and/or sleep diagnosis; and 10) presence of fair or poor physical health, as assessed by the General Medical Health Rating Scale.
Control subjects were individuals who had previously participated as healthy volunteers in one of the co-investigator's (UM) research studies and who were matched to mTBI subjects for age, gender, and BMI. Notably, all of the healthy-controls met criteria for the present study. In particular, they were healthy, drug-free individuals who were recruited from the community with IRB-approved advertisements and IRB-approved telephone screens. All control subjects had undergone thorough medical and psychiatric screens, and adaptation and “baseline” sleep studies using the same methods specified in the current study.
Evaluations
After determining that subjects met inclusion criteria and providing informed written consent, subjects were admitted to an inpatient clinical research unit where they underwent two consecutive overnight PSG studies. The first night served as an adaptation night to enable acclimation to the sleep-lab setting; sleep PSGs from the second night were used in data analyses. To maintain a controlled environment and prevent introduction of potential confounds, participants were not allowed to go home after the first PSG.
Polysomnogram (PSG)
PSGs were performed at the Johns Hopkins Bayview Clinical Research Unit, which is a component of an Institutional Clinical Translations Research program. Standard sleep montages were used for electrode placement. Also, heart rate was continuously monitored by an ECG, and arterial oxyhemoglobin saturation (SaO
2) was recorded by finger oximetry, thoraco-abdominal movement was assessed by abdominal strain gauges, and airflow was measured by nasal-pressure transducer and thermistor. Leg movements were measured using electrodes placed on the anterior tibialis muscles. The baseline overnight PSG was visually scored by certified polysomnographers from the Johns Hopkins Sleep Disorders Clinic, using the American Academy of Sleep Medicine (AASM) standards.
2 PSG staging was then utilized to define REM and NREM periods, according to Feinberg and Floyd,
3 except that there was no minimum length of time imposed to classify a REM period.
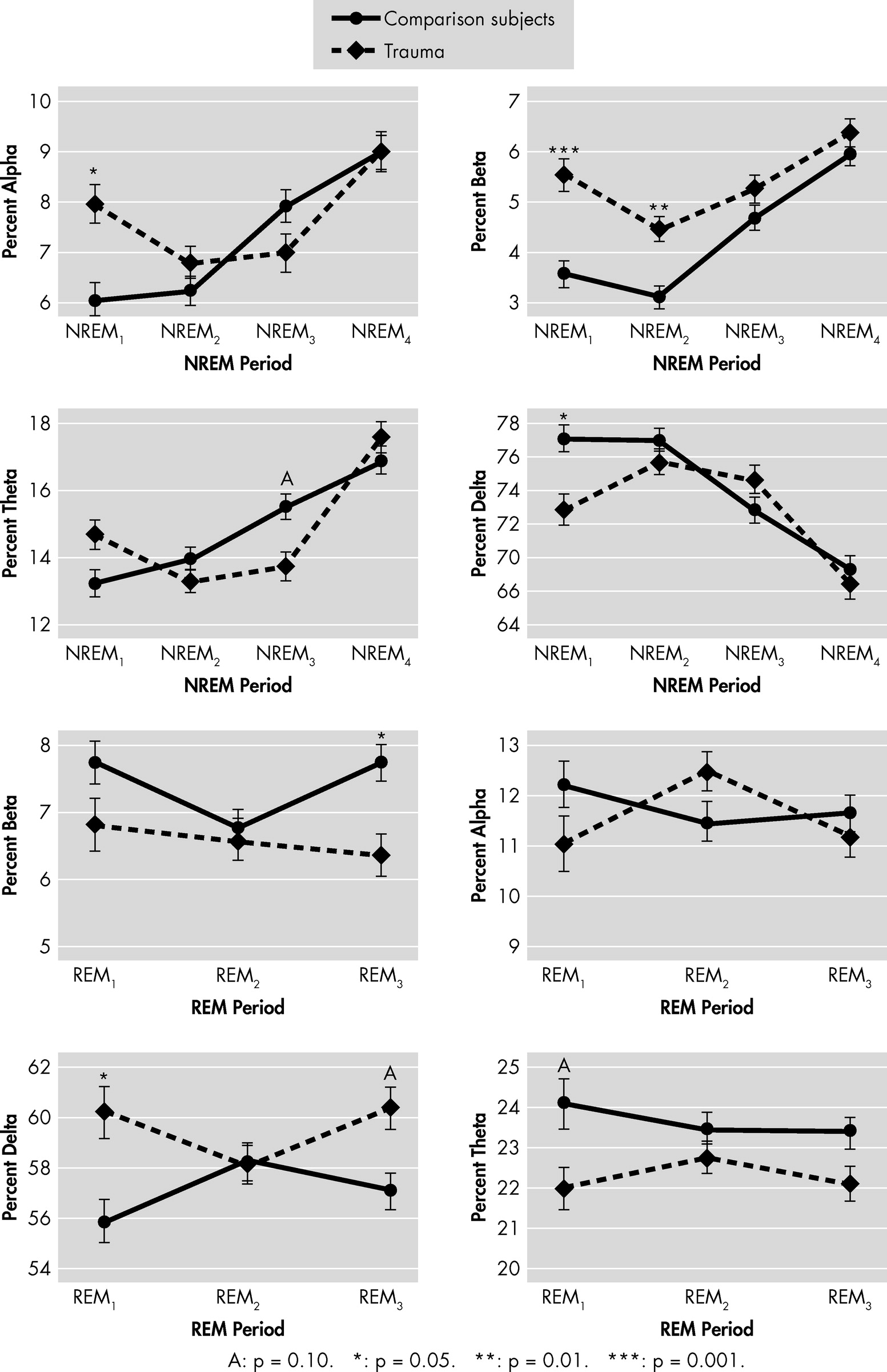
Power Spectral Analysis (PSA) of EEG
Spectral analysis of the C3-A2 EEG recording was analyzed using the discrete fast-Fourier transform.
4 The spectral-frequencies bands were categorized into: δ (0.8 to 4.0 Hz); θ (4.1 to 8.0 Hz); α (8.1 to 13.0 Hz); and β (13.1 to 20.0 Hz). Synchronizing the epoch-by-epoch spectral estimation with the sleep stages (and with REM and NREM periods) allowed for comparisons of power spectra within specific sleep stages. It also allowed for removal of wake epochs and various artifacts in the EEG signal. Artifacts were determined by visual inspection by plotting the spectral bands against the epoch of sleep for each participant. Spectral bands were plotted with a five-epoch moving average. An overlay plot of the AASM sleep scores was used to assess locations of irregularities in the EEG signal (e.g., a large amount of beta-frequency activity in Stage 3 sleep, little-to-no delta-frequency in Stage 3). If an irregularity was identified, the entire montage of signals during that time-period was viewed in detail to confirm artifacts.
Statistical Analysis
Between-group comparisons of sleep-architecture measures were performed by multiple linear regression, adjusting for race and age. Spectral power measures in each sleep stage were evaluated for between-group differences by use of a generalized linear mixed model (GLMM) to account for repeated measurements within subjects. Spectral power was also analyzed for between-group temporal trends within REM and NREM periods independently, with an autogressive
1 (AR
1) covariance-structure. The GLMM procedure used Group, Period, and Group × Period as parameters in the model, again adjusting for race and age. When a main effect of Group or a Group × Period interaction was found, the least-squares means of fixed effects were compared at individual periods to determine the nature of these findings. Statistics were conducted using MATLAB (The Mathworks, Inc., Natick, MA) and SAS (SAS Institute Inc.; Cary, NC).
DISCUSSION
The current pilot study is the first to assess the sleep polysomnograms and EEG power spectra (PS) in acute mTBI as a potential marker of brain injury. The major finding is that, as compared with matched healthy-control subjects, mTBI patients had abnormalities in sleep EEG PS.
These preliminary data suggest that disturbances in sleep may provide a tool in the armamentarium of clinicians who diagnose and treat patients with mTBI. Although the basis for abnormalities in sleep in patients with mTBI cannot be determined using the current methods, a likely cause is diffuse axonal injury involving several brain circuits that are known to play important modulatory roles in normal sleep mechanisms.
5–7There is only one other study, by Tebano et al.,
8 that has examined electroencephalographic (EEG) spectral analysis in patients with minor and moderate head injuries in the acute trauma period. The study found that subjects with head injury, as compared with healthy controls, had an increase in slow alpha-wave power and a decrease in fast alpha- and beta-wave power. The alpha power dysregulation seen in this study partially supports our study results. However, unlike our study, which analyzed power spectra on sleep EEGs, Tebano et al.
8 only recorded EEGs in the morning for 3 hours. There is no other study that has examined sleep PSGs or sleep power-spectra in acute mTBI patients. This is an important area of research, because PSGs and power-spectra have been found to be useful diagnostic and prognostic markers of moderate and severe TBI.
9–11These studies underscore the importance of examining the macro- and microarchitecture of sleep in mTBI patients, given that, currently, there are no quantifiable objective diagnostic or prognostic markers of injury. The current feasibility study is the first step toward this process; an attempt to identify a diagnostic marker of mTBI.
Clearly, mTBI is not a uniform diagnosis. The neuropathology associated with mTBI will depend on the nature and location of the underlying injury. Sleep PSGs and limited PSA analyses may provide a somewhat crude biomarker of injury as an initial step in the diagnostic work-up. If abnormalities in the PSG and PSA are observed, more detailed PSA methods, using electrodes at multiple locations (e.g., frontal, temporal, occipital) could be further used to localize the site of the most severe lesions. Additional research will be required to determine whether the location and severity of sleep EEG abnormalities can be used as a predictor for longer-term disability.
The major limitations of the present pilot study are its small size and the rigid criteria used to select mTBI subjects. The small sample size limits the power of our conclusions and, in theory, could be associated with a false-positive outcome. Also, given the number of variables examined, there is the potential for false positives due to multiple comparisons. However, because this is a hypothesis-generating study, we have used a significance level of p<0.05, rather than incorporate a Bonferroni correction. The rigid inclusion criteria may also limit the generalizability of our findings. However, if subjects with previous psychopathology, substance abuse, or previous brain injury had been included, these confounds would have severely limited our ability to arrive at meaningful results. Finally, even though people with pre-TBI sleep disturbances and/or sleep diagnoses were excluded from the study, it is possible that some of them who may have had preexisting sleep disturbances and were not aware of it were included in the study. Also, the clinical significance of the PSG findings of sleep apnea and/or periodic leg movements is not clear, given that none of the mTBI subjects reported significant difficulties in sleep in the pre- or immediate post-TBI period.
The current findings must be considered only preliminary. Clearly, the nature and neuroanatomy of brain injury after mTBI will dictate the nature of the subsequent sleep disturbance.
Acknowledgments
We thank Dr. Naresh Punjabi for his consultative advice on power spectral analyses, Francis P. Sgambati for assistance with sleep data analyses, and the CRU/ICTR nursing staff for their participation in the execution of these studies.
This work was supported by K23 MH 066894 from the NIMH (Rao) DA16563 (McCann); and NCRR grant M01RR002719 (Ford). Dr. Rao has a grant from Forest to conduct a clinical trial in post-TBI depression and a grant from Pfizer to conduct a clinical trial in post-stroke anxiety. Dr. McCann served as a speaker for Jazz Pharmaceuticals in 2008 and received $14,000 for speaking engagements. She also served as an expert legal witness in an ongoing legal case and has received $18,000 for her services. Ms. Bergey is a research assistant on many studies supported by pharmaceutical companies (Merck, Forest, Elan, Pfizer, Eli Lilly).