Auditory verbal hallucinations (AVHs), the perception of voices in the absence of auditory stimuli, are common and distressing symptoms reported by 50%–80% of patients with schizophrenia.
1 However, the results in a number of imaging and electrophysiological studies on the origins of AVH, are not consistent, and the underlying pathophysiology still remains unclear. Neuroimaging studies have associated occurrences of AVHs with activation of diverse brain regions involved in speech generation, speech perception, and verbal memory.
2–7 Many of these studies have found prominent activation during AVHs in the right as well as left hemisphere,
4,5 with considerable intersubject variation in the cortical areas involved.
6A “symptom-capture” approach attempts to explain AVHs by imaging the dynamic changes of brain functioning in terms of EEG activity, blood flow, and metabolism, targeting the “envelope of the symptom,” using EEG, functional MRI (fMRI), or positron emission tomography (PET), in periods associated with the appearance of auditory hallucinations. This approach was first introduced in 1995
2 as a method for capturing hallucinations during
[15]O PET scanning. Although this approach is conceptually simple, a number of confounding factors related to the ability of the patient to precisely report the initiation and completion of the hallucinatory experience affect the temporal location of the hallucination. Nevertheless, a number of investigators have used this approach successfully, reporting that auditory hallucinations are associated with activation of speech-production areas, primary and secondary auditory cortices, and various polymodal association cortices.
3,5 From an electrophysiological point of view, limited research data have given support to the central auditory-processing deficit model. These studies showed that AVHs are associated with increased beta-frequency oscillations generated in speech-related areas.
8 Moreover, increased α-band relative coherence between the left and right superior temporal cortices has been reported during AVHs.
9The aim of the present study was to detect possible changes of EEG properties temporally anchored in the vicinity of the hallucinatory experience in schizophrenic patients with persistent AVHs. Thus, we exclusively focused on the brain functioning associated with the experience of AVHs and did not extend the focus to a description of global or state EEG characteristics related to schizophrenia in general. Under this experimental design, the EEG phase-stability of selected brain sites was considered as an expression of the functional coupling and was assessed in the presence of AVHs. Two different computations of phase synchrony were applied in our EEG recordings: 1) the band-specific synchrony (BSS), which expresses the spatial-temporal average of the instantaneous neuronal coupling; and 2) the frequency-specific synchrony (FSS), which focuses on the temporal mode of the synchrony associated with the experience of AVHs, as reported by the patients.
The study compared two patient groups suffering from schizophrenia: 1) patients with auditory verbal hallucinations; and 2) patients without hallucinations; with a third group of normal subjects. The authors examined BSS and FSS in the broad frequency region of α EEG band (6–13 Hz).
METHOD
A group of 17 schizophrenia patients and 16 normal-control subjects participated in the study. After a detailed description of the experimental protocol, all subjects gave written informed consent, and we obtained University Mental Health Research Institute Ethics Committee approval. The inclusion criteria were the following: 1) age between 18 and 45 years; 2) right-handed; 3) no alcohol and drug abuse in the last 5 years; 4) no neurological illness or head trauma that would result in an abnormal EEG; 5) no antiepileptic drugs; and 6) no alcohol use in the last 24 hours. Normal-controls had no Axis I psychiatric disorder. Two patients' data were excluded from the analysis because of artifacts related to limited cooperation during the experimental procedure. All patients were recruited from Eginition University Hospital and fulfilled the criteria for schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition–Text Revision (DSM-IV–TR; 2000). The Positive and Negative Symptoms Scale (PANSS) was used for assessment of symptoms. The detailed characteristics of the hallucinations were assessed with the Psychotic Symptom Rating Scales–Auditory Hallucinations Rating Scale (PSYRATS–AHRS).
10 All clinical data were collected 1 day before the EEG recording.
Two groups of patients participated in the study; first: the SCZ-AVHs group consisted of 8 patients with drug-resistant spontaneous AVHs (4 men, 4 women; mean age: 36 (SD: 7) years; duration of illness: 15.5 (SD: 6) years; mean PANSS score: 70 (SD: 6); 6 of these patients were medicated with risperidone (mean dose: 7 mg/day) and 2 with amisulpride (mean dose: 800 mg/day); and second: the SCZ group, consisting of 7 patients who, at the time of enrollment, did not exhibit AVHs, as a result of effective antipsychotic drug treatment, (3 men, 4 women, mean age: 30 (SD: 9); duration of illness: 13 (SD: 8) years; mean PANSS score: 68 (SD: 8). Four of these patients were medicated with risperidone (mean dose: 7 mg/day), two with amisulpride (mean dose: 600 mg/day), and one with aripiprazole (dose: 25 mg/day). All participants were selected from a larger sample of subjects suffering from schizophrenia, but who had the ability to cooperate in the experimental procedure. The Control (NOR) group consisted of 16 subjects (8 men, 8 women); mean age: 31 (SD: 6) years.
Subjects were seated in a light- and sound-attenuated, double-skin Faraday cage. Electrodes (Fp1, Fp2, F7, F3, Fz, F4, F8, FT7, FC3, FCz, FC4, FT8, T7, C3, Cz, C4, T8, TP7, CP3, CPz, CP4, TP8, P7, P3, Pz, P4, P8, O1, Oz, O2) were placed on the scalp, using a standard cap. Recordings of the horizontal-plane eye-movement potentials were made by two electrodes fixed 1 cm bilateral to the outer canthus of each eye. The skin resistance of each electrode was kept at ≤5 kΩ for the entire session. The participants were instructed to report any experience of auditory hallucination. This was achieved by pressing a miniature optical switch with the middle finger of their dominant hand to indicate the onset and the duration of any experienced AVH. In order to validate our findings, the data obtained from the SCZ-AVHs group were compared with those obtained by the NOR and SCZ, where the participants were instructed to press the button in a voluntary basis, without any previous prompt. The above procedure was applied both during eyes-open (E-OP) and eyes-closed (E-CL) conditions. The EEG signals were acquired by a Synamps (Neuroscan Labs) amplifier module sampled at 500 Hz. The phase stability of selected sites was computed by the following equation:
where
C expresses the temporal coupling of two preselected brain sites, α and
b as a function of frequency
f and time
t;
w is the duration of the time window, varied from 50 to 200 msec; and ϕ is the phase of the convolved signal with the wavelet. In practice, the above formula was computed in a number of overlapped phase segments corresponded to a period of 1.5 sec preceding the end of the mark that denotes the end of the hallucination constituting, in this way, the parameter FSS. In order to evaluate the statistical significance of our findings against the background phase-difference fluctuations, FSS values obtained from original EEG signals were compared with those obtained from surrogate data-sets. The results gave the amount of coupling as a function of time, with value of 1 indicating the higher and 0 the lower coupling. This analytical technique is sensitive in the detection of frequency-related changes of the phase-synchrony and is accurate enough to study phasic-synchronous activity in single trials.
11 Also, in order to investigate the spatial characteristics of α-band synchrony in association with auditory-hallucination states, a coupling index
12 was computed across the reference and the remaining electrodes. The spatial-temporal average of this index constitutes the band-specific synchrony illustrated in
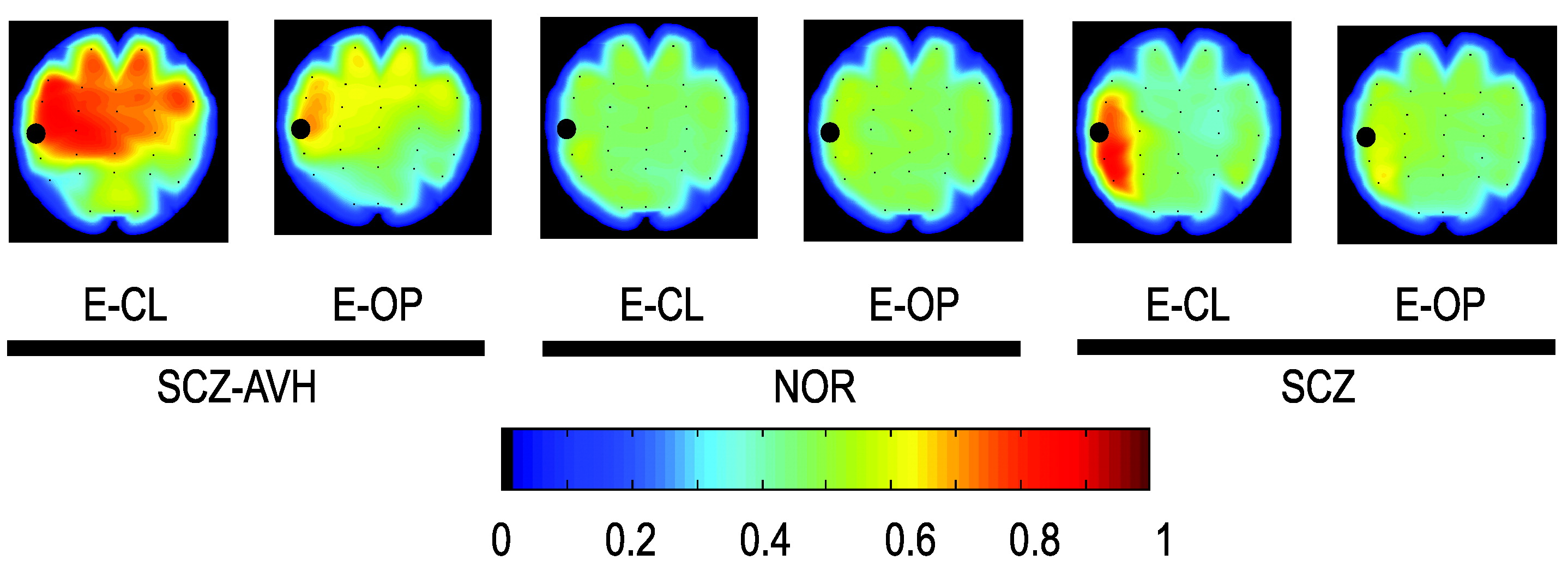
Figure 1.
RESULTS
A total number of 472 hallucinations were reported by the SCZ-AVHs patients during the recording procedure. The mean duration of hallucinatory experience was 8.1 (SD: 9) sec under the E-CL and 5.3 (SD: 6.5) sec under the E-OP experimental condition. The rate of acousmata (hallucinations/min) under the E-CL condition (mean: 6.62 (SD: 1.9) was found to be significantly higher than the rate of acousmata in the E-OP condition (mean: 4.1 [SD: 2]); ANOVA (F[1,14]=4.73; p<0.05). Time-averaged BSS computed from EEG activity in SCZ-AVHs, SCZ, and NOR subjects (mapped in
Figure 1) specifically showed spreading phase-coupling in the SCZ-AVHs group, intra- and inter-hemispherically, at left and right frontal and temporal areas under both eyes-closed and eyes-open condition, with this finding more pronounced under the eyes-closed condition. The BSS distribution of the NOR as well as the SCZ group, under the same experimental condition, did not show persistent synchrony. In this manner, BSS results showed the general tendency to brain coupling during periods with sustained AVHs, independently of the moment that the hallucinatory events occurred.
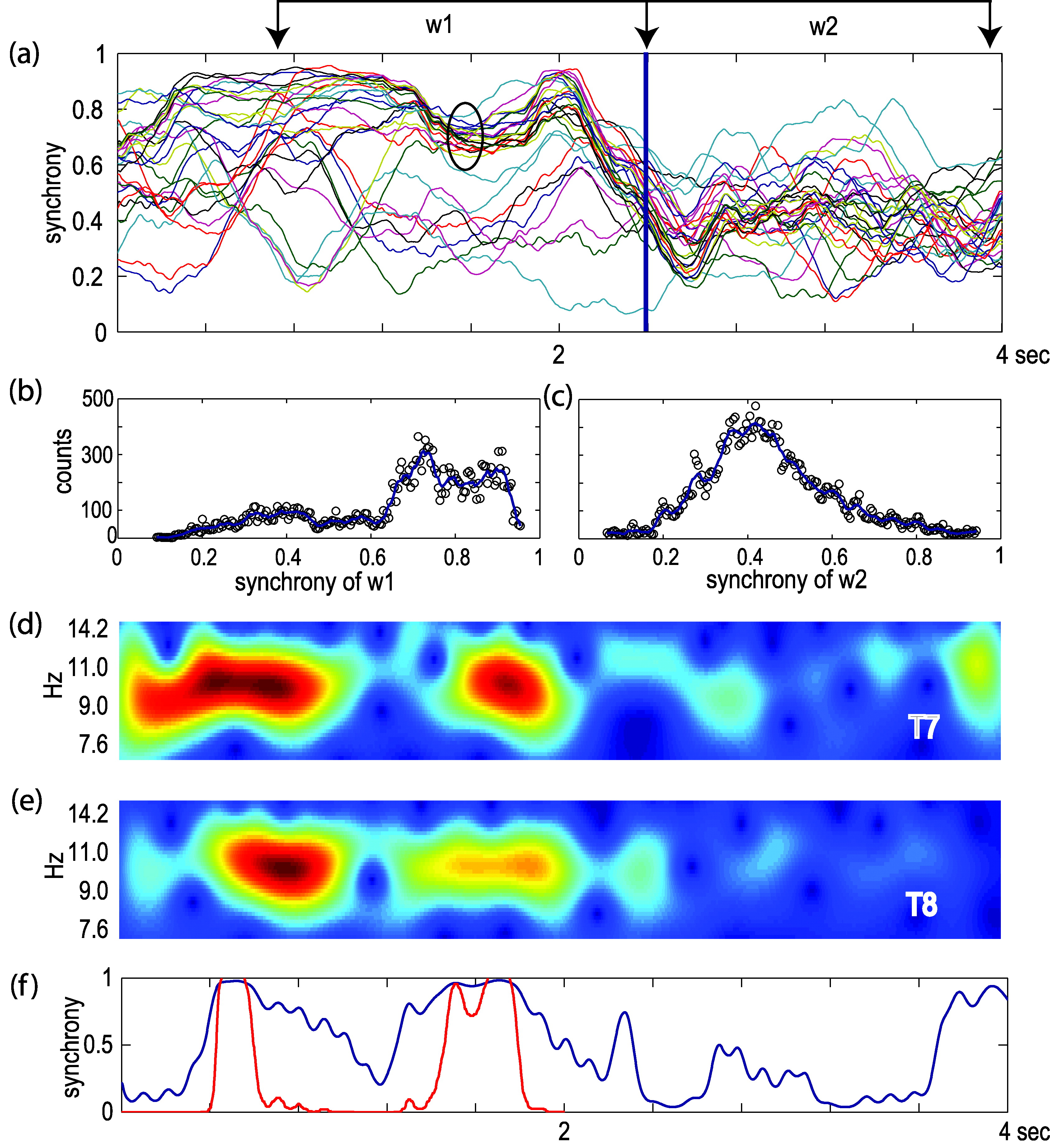
Following the “symptom-capture” approach, we attempted to relate AVH experiences with synchrony fluctuations before and after the end of the AVH w1 and w2 windows respectively (see
Figure 2 [a]). As shown in
Figure 2 [a], the synchrony traces converged during the AVH, while they diffused after the end of the experience (blue vertical bar). The encircled cluster of traces includes the synchrony of the anterior part of the brain of both hemispheres with respect to the T7 electrode. The distribution of synchrony values regarding the w1 and w2 time windows was used as a measure to characterize the dynamic mode of coupling observed in the frontal brain areas during an AVH event. The observed shift of synchrony distribution to higher values before and lower values after the end of an AVH, was a common finding in the majority of the cases examined (
Figure 2 [b], [c]). In the above-illustrated case, the underlying oscillatory activity recorded at T7 and T8 showed increased amplitude, accompanied with high synchrony envelopes, before the specific AVH, indicating strong inter-hemispheric coupling (
Figure 2 [d]–[f]).
The authors subjected to statistical analysis 40 synchrony cases obtained from SCZ-AVHs in whom the inter-hallucination interval was greater than 8 sec, with 40 cases (“dummy” pressings) of SCZ and NOR groups. In the SCZ-AVHs group, the maximum synchrony values were estimated within a 1.5-sec window before the end of the hallucinations as these were denoted by the subjects. In the same way, the maximum synchrony values of the SCZ and NOR groups were estimated with respect to the timing of dummy pressings. Maximum synchrony values of the SCZ-AVHs group (mean: 0.63; SD: 0.7) were found to be significantly greater than those of the NOR (mean: 0.29; SD: 0.19) as well as the SCZ group (mean: 0.26; SD: 0.25; ANOVA (F[1,78]=69.23; p<0.001; and F[1,78]=56.55; p<0.001), respectively. No differences were found between the SCZ and NOR groups ANOVA (F[1,78]=0.43; NS).
Likewise, significantly longer latency values between the button-release and the maximum synchrony were found between the SCZ-AVHs group (mean latency: 760 [SD: 293] msec) and the NOR (mean latency: 450 [SD: 411] msec) as well as the SCZ (mean latency: 453 [SD: 358] msec) group; (ANOVA: F[1,78]=7.95; p<0.01) and (F[1,78]=142.72; p<0.01), respectively. No differences were found between the SCZ and NOR groups (F[1, 78]=0.014; NS). The above findings indicate that concentration of synchrony peaks converged in a limited time-window before the end of the button-pressing in the case of SCZ-AVH group. In the SCZ and NOR groups, the absolute values of the observed synchrony peaks, as well as the latencies, were widely distributed, suggesting that these peaks were not causally related.
DISCUSSION
Spontaneous EEG oscillations at various frequencies exhibit transient phase-concordance, which systematically relates to behavioral and or experimental conditions. These EEG oscillations seem to play an important role in the spatial characteristics of the neuronal assemblies involved in specific processes,
13 and their extent provides information regarding the neuro-cognitive processes involved in specific psychiatric symptoms such as AVHs.
The evidence that α-band activity is the most pronounced EEG oscillation between the temporal regions bilaterally,
14 along with our finding in which a significant increase in the frequency of AVHs has been observed during the eyes-closed, versus the eyes-open, condition raised the question of possible involvement of α oscillations in the inception of a neurocognitive state in which the production of AVHs can be experienced by the subject. This led us to focus on analysis of the phasic characteristics of a broad α EEG frequency region in the auditory-related cortices of hallucinatory patients. In this line of evidence, our results further contribute to the understanding the specific role of α oscillations recently reported in 2001 and 2007, respectively.
15,16 In the present study, we found 1) An increased rate of AVHs experienced by the SCH-AVH subjects during the E-CL, as compared with the E-OP experimental condition; 2). An increased phase-coupling of α-band (BSS analysis) in the SCZ-AVH group distributed intra- and inter-hemispherically in the anterior brain areas under both E-CL and E-OP conditions; 3) This was more profound under the E-CL condition; 4) A statistically significant increase of α-band FSS maximum synchrony values in the SCZ-AVHs group, as compared with the NOR and the SCZ groups at the T7–T8 electrode pair inter-hemispherically.
These synchrony values were observed in a time-window related to the report of the hallucinatory experience. In the same way, significant difference in latency was found between the SCZ-AVHs group and the SCZ/NOR groups between the AVH report and the peak of synchrony observed. These results support the assumption of an α oscillation involvement, by means of phase-coupling, in the processes underlying the production of AVHs. In α-band amplitude studies, α oscillations have been thought to reflect idling
17 or active inhibition of task-irrelevant brain circuits.
18,19 However, recent data on α amplitude and, in particular, α phase-dynamics, posit a direct and active role for α-band rhythmicity in the mechanisms of attention and consciousness. This view is supported by the positive correlation of the α amplitude with short-term memory and working-memory load
18,19 and task difficulty.
20 Furthermore, α oscillations can phase-lock between widely-separated cortical regions
21,22 and, thus, form functional large-scale networks.
23Enhanced α-band synchrony in the fronto-parietal network is associated with the execution of cognitive tasks.
22 However, in studies reporting strengthened α-phase synchrony, some show a simultaneous amplitude increase,
24 whereas others show an associated amplitude suppression.
22,25 In our study, high-amplitude α oscillations were associated with long-distance phase-coupling in time-windows closely related to the hallucinatory experiences in the symptom-capture paradigm. Our results illustrate increased intra- and inter-hemispheric temporal coupling during persistent hallucinatory states. This finding is in agreement with observations made in 2005 in an EEG coherence study,
9 where an abnormal increase of the inter-hemispheric functional connections between the auditory cortices was observed during the experience of AVHs.
During phase computations, the contribution of a volume-conduction component should be carefully considered, particularly between the proximal electrode sites. In this study, the long-distance synchronization found excludes, by definition, the influence of this factor. Moreover, in the FSS analysis, the synchrony peaks found were temporally engaged with the marked AVHs, a fact that reduces the effect of phase leak due to the volume conductance. Another issue that should be considered in our methodology is the confounding factors related to the ability of the patient to precisely report the initiation and completion of the hallucinatory experience. However, in order to limit subjectivity errors, the authors carefully selected patients with insight, high level of education, and good social functioning, to improve the cooperation during the experimental procedure. Auditory verbal hallucinations are considered to be complex features, and, as such, they reflect abnormal functional connectivity in multiple related regions in both intra- and inter-hemispherical sites. Since brain functional connectivity is defined primarily by phase-integration; our findings indicate that acousmata are related to abnormal neuronal synchrony that implies erroneous and, in a sense, autonomous functional connectivity.