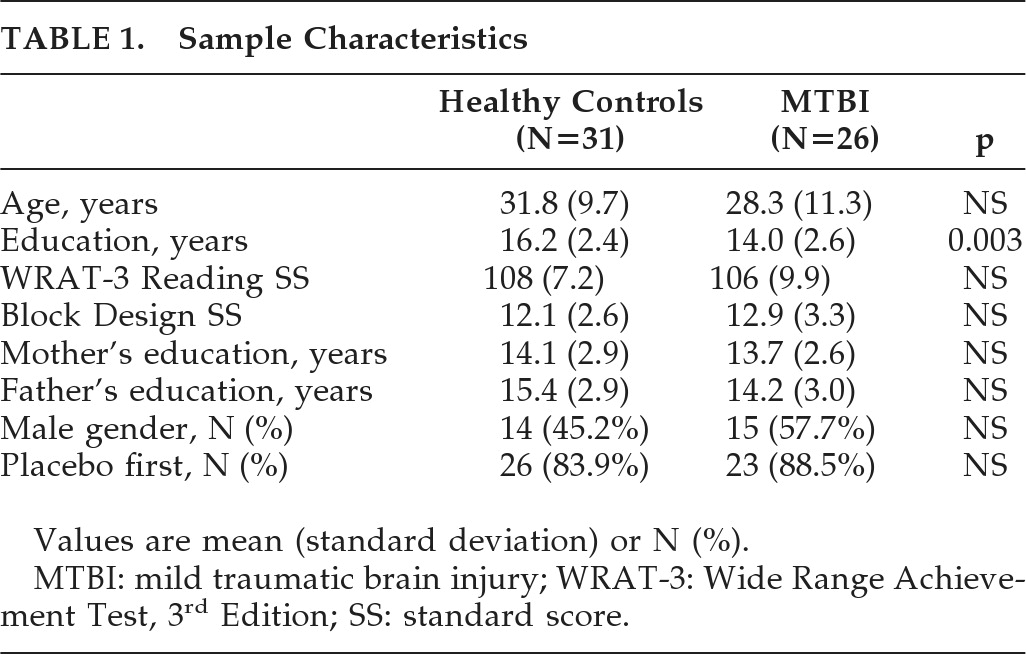
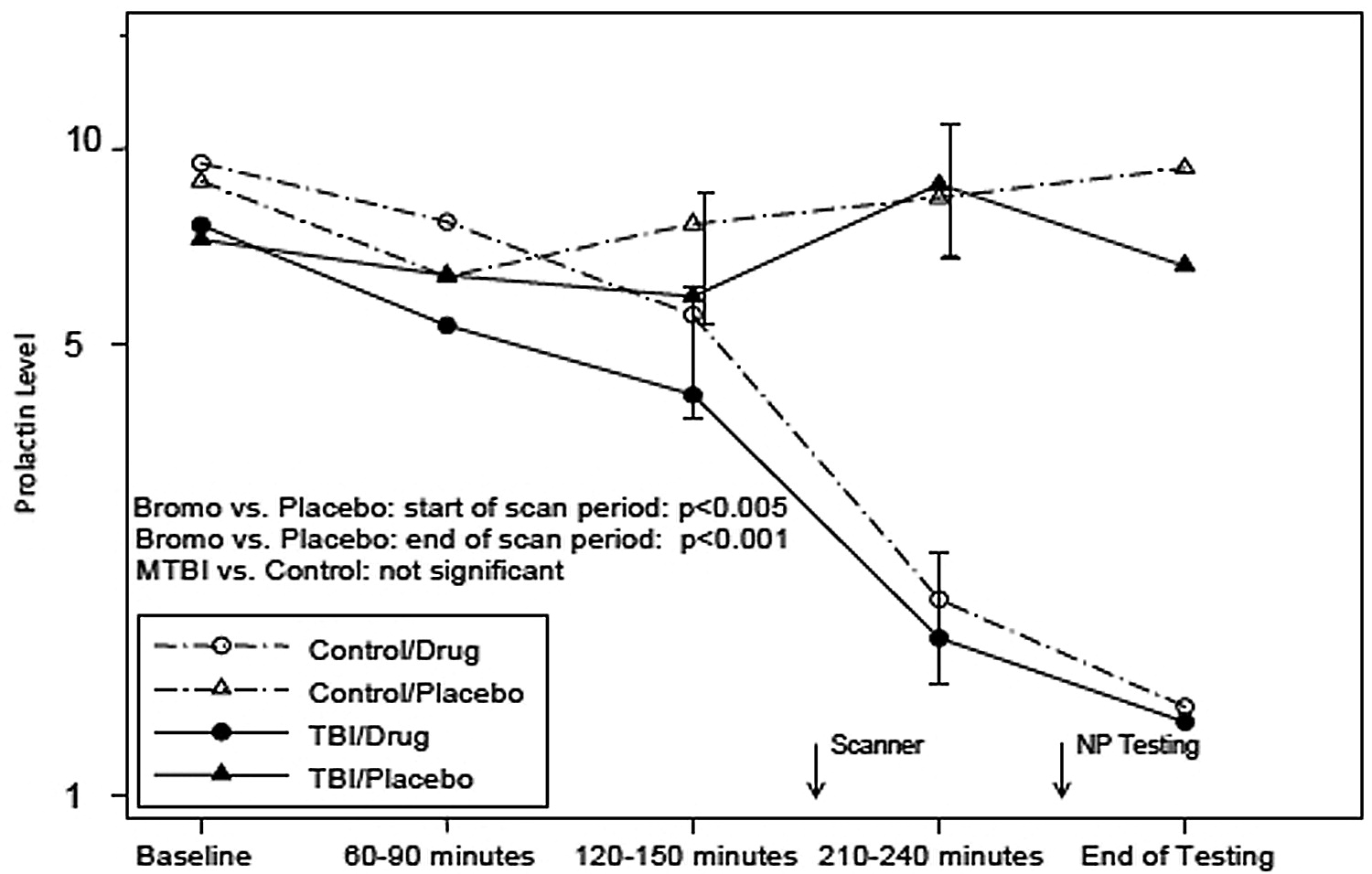
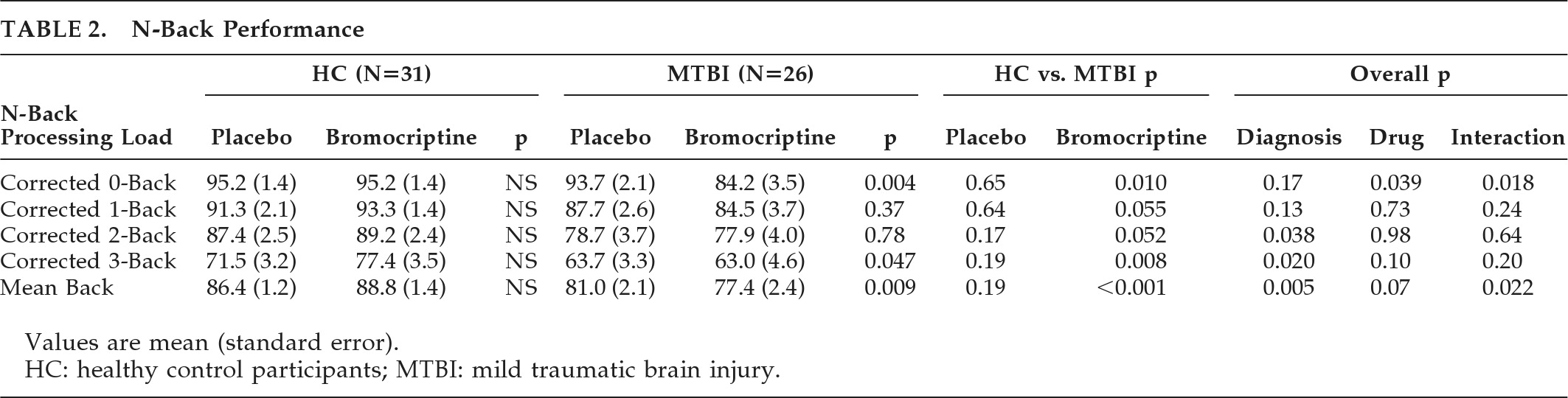
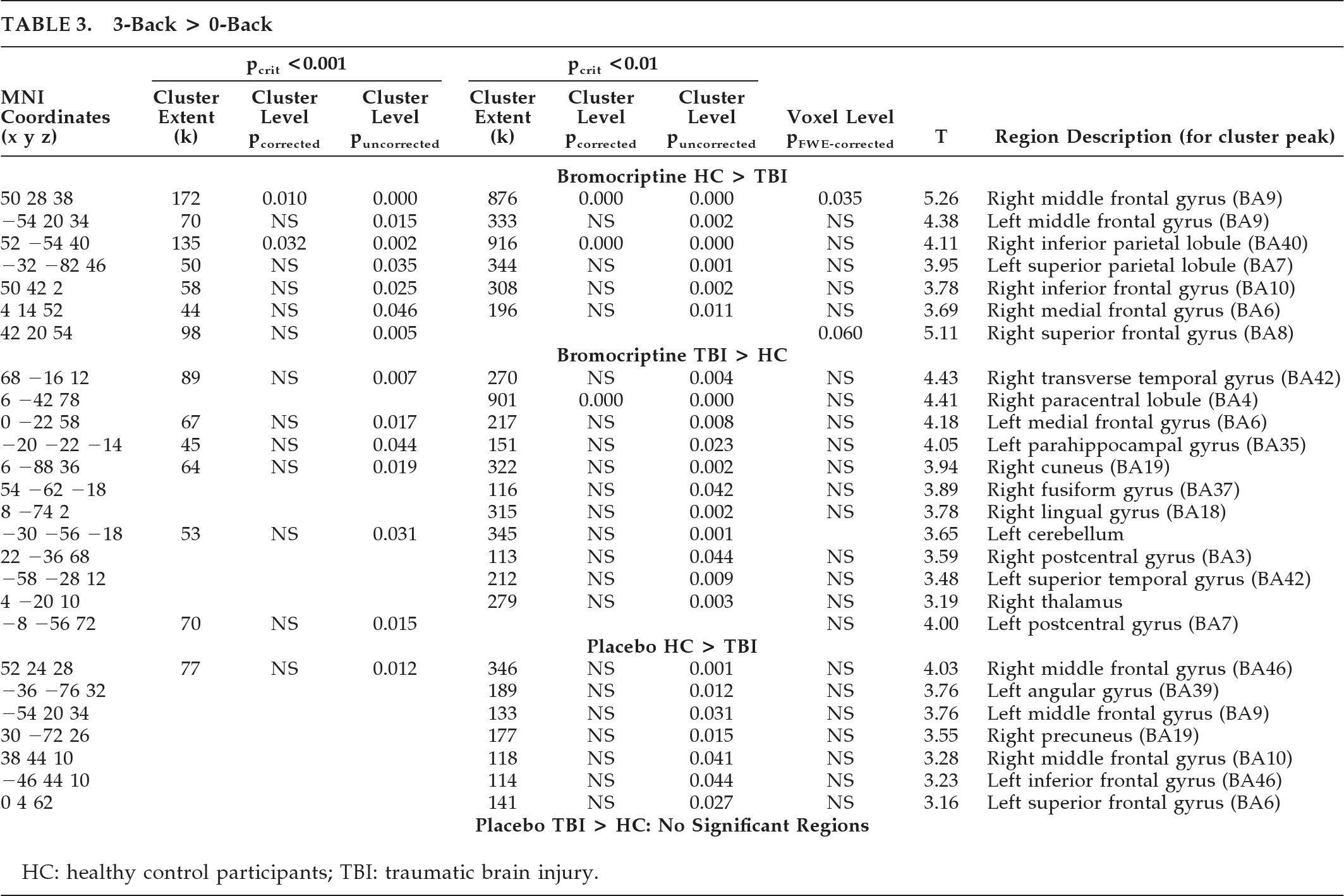
Initial and persistent cognitive deficits are the most common complaints after TBI
1 and are the major hindrance to return to baseline functioning.
2 There are several predictable areas of cognitive impairment after TBI;
3–6 these include working memory (WM), which is the ability to hold information in mind and to manipulate that material in light of incoming information. We previously used functional MRI (fMRI) to demonstrate that 1 month after mild TBI (MTBI), performance on a moderately difficulty WM task was associated with significantly greater cerebral activation (“compensatory activation”) and increased memory complaints, despite the fact that WM task performance was similar to that seen in controls.
7 We subsequently showed that MTBI patients were unable to further increase brain activation with a higher WM processing load.
8 We interpreted these results as evidence for abnormalities in the activation and allocation of WM processing resources after MTBI. In essence, patients were “working harder” to achieve the same results with a moderately difficult task, and thus had fewer additional processing resources to recruit during a more difficult task condition. Other groups have subsequently shown alterations in cerebral activation after MTBI.
9,10The mechanism underlying these findings has not been addressed. A significant body of work suggests that catecholaminergic mechanisms play important roles in WM functioning, especially in the prefrontal cortex (see Arnsten et al.
11). We have suggested
12 that these abnormalities play a role in abnormal activation and allocation of WM processing resources after MTBI. Although it is difficult to test this hypothesis directly, it is possible to use pharmacological challenges to probe whether individuals with MTBI differ in their response to catecholaminergic agents. Altered behavioral (cognitive) and neurophysiological (task-related cerebral activation) responses to a catecholamine agonist would provide indirect support for our hypothesis. In this study, we hypothesized that individuals with MTBI would show altered cognitive and cerebral activation patterns in response to the dopamine D
2 receptor agonist bromocriptine.
DISCUSSION
Experimental results indicate that individuals with MTBI have altered cognitive and cerebral activation patterns in response to the dopamine D
2 receptor-agonist bromocriptine, consistent with the main study hypothesis. Of particular interest is that an overall N-back performance indicator, the mean back-accuracy score, showed a significant (p<0.02) Diagnosis × Drug condition interaction. Post-hoc analysis of this interaction shows this was due both to improvement in the HC group and poorer performance in the MTBI group on bromocriptine. Furthermore, the pattern of cerebral activation in response to bromocriptine differed between the two groups. Increased activation in WM circuitry in the HC group, including bilateral frontal and parietal regions, was more pronounced on bromocriptine, and greater right middle frontal activation also correlated with improved task performance across both groups, suggesting some functional significance to the finding. The increased activation of this region while on bromocriptine in HCs, coupled with the improved performance in HCs as compared with the MTBI group, raises the possibility that bromocriptine facilitates selective recruitment of task-related processing resources in the healthy brain. In contrast, MTBI patients on bromocriptine showed increased activation in brain regions not typically associated with WM processing, suggesting an altered neural response to bromocriptine challenge. This may suggest that the MTBI group is less efficient at deactivating brain regions outside of task-related neural circuitry during WM processing, or that there is compensatory activation of these resources in an effort to maintain WM performance, which, in this cohort, was unsuccessful. Taken together, these findings are consistent with the hypothesis that subtle changes in the central dopaminergic system may contribute to previously described alterations in WM processing after MTBI.
7,8,25Our results differ in some ways from related studies. For example, improved performance on a dual-task paradigm while on bromocriptine was noted in 24 individuals with TBI.
30 However, participants in that study were of mixed injury severity, were studied at variable intervals after injury, and performed a different task from the one used in our study. Subsequent studies did not replicate the previous finding and, in fact, noted a trend for bromocriptine to be associated with poorer performance on a variety of attentional measures.
31 Other discrepant findings have been reported in response to dopamine agonists,
32 and dose–response effects with dopamine agonists have been noted: low doses can facilitate WM, whereas higher doses can impair performance.
33–35 It is possible that those with MTBI are more sensitive to the effects of dopamine agonists, and thus perform more poorly or do not show the same improvement as HCs at equivalent doses of bromocriptine. There may also be a biphasic pharmacokinetic response with bromocriptine. Pizzolato et al.
36 suggested that initial effects of bromocriptine can be inhibitory, whereas later effects can be excitatory. Our study used different ingestion-to-fMRI intervals from previous studies (about 1.5 hours, versus about 2.5 hours). Previous work in rodents, using a controlled cortical impact model of TBI,
26,27 suggests that TBI-associated changes in levels of frontal and basal ganglia dopamine transporter are more pronounced in males. Also, there may be gender-related differential responses to interventions such as environmental enrichment, perhaps related to neuroprotective effects of female sex hormones.
27 This raises the possibility that similar gender differences might exist in human TBI, and thus response to dopaminergic challenges might differ in men and women. Although we did not find such differences in either the cognitive outcome or the BOLD response, it is possible that differences might be more apparent with a larger sample size. It is also interesting that we did not find differences within the MTBI group as a function of presence or absence of LOC at the time of injury. However, report of LOC in the MTBI group is not always reliable, and, even in those who did report LOC, the mean duration was brief (4.8 minutes). Again, larger sample sizes might reveal such differences.
The mechanism of dopamine system dysregulation is not clear. MTBI is associated with alterations of catecholaminergic systems (including dopamine) that can be prolonged, may be associated with alterations in catecholaminergic receptor density in damaged cortical areas, and can impair catecholaminergic function after trauma.
37,38 The studies by Wagner et al.
26,27 suggest that, at least in rodents, TBI is associated with reduced dopamine transporter levels in the striatum. Neuronal damage in the region of the anterior frontal cortex might also contribute to the changes in brain activation, with a relative absence of anterior frontal activation seen in the MTBI group. Although it is clear that neuronal loss does accompany MTBI,
39–41 it is generally considered to be greatly reduced in extent and significance relative to that accompanying more severe injuries.
Alternatively, dopaminergic dysregulation may be a downstream effect of dysfunction of other neurotransmitter systems, such as the cholinergic system. There is a significant body of work in animals and humans suggesting that TBI results in alterations to the cholinergic system (for reviews, see DeAngelis et al.,
42 Verbois et al.,
43 and Arciniegas
44). Given the reciprocal hippocampal–prefrontal connections and their interplay in the regulation of memory,
45 it is possible that subtle changes in cholinergic tone, stemming from excitotoxic injury to highly vulnerable regions of hippocampal cortex, result in apparent changes in prefrontal dopaminergic regulation.
Several limitations should be considered in the interpretation of these findings. We intentionally did not include MTBI participants with significant medical and psychiatric disorders; therefore these results may not generalize to all individuals with MTBI. This MTBI group had very mild injuries, and the results may not apply to those with moderate and severe injuries. It is also important to point out that the regions of increased activity evident on bromocriptine do not necessarily indicate the exact locations of dopaminergic effect. Although BOLD fMRI activation in these areas indicates local increased cerebral blood flow and metabolic activity, bromocriptine administration could indirectly modulate this activity through striatal or other interconnected subcortical or cortical sites. Also, given the behavioral/performance differences on bromocriptine between the HC and MTBI groups, we cannot rule out the possibility that the area of increased activation in the right middle frontal region (see
Figure 2 and
Table 3) may modulate other behavioral effects, which then secondarily affect WM performance. However, the fact that this region is embedded within a neural network known to play a key role in verbal WM circuitry is consistent with a drug-related WM modulatory effect.
Despite these limitations and caveats, the current results remain most consistent with the conclusion that MTBI is associated with subtle dysregulation of frontal dopaminergic systems in the first 4–6 weeks after injury and that simple augmentation strategies with a dopamine agonist that affects predominantly D2 receptors may not improve cognitive functioning. Further studies are needed to clarify the effects that different dosing strategies, severity of injury, and the injury-to-treatment interval may all have on outcome.
Acknowledgments
Location of Work: Section of Neuropsychiatry and Brain Imaging Laboratory, Departments of Psychiatry, Radiology, Community and Family Medicine (Biostatistics), Emergency Medicine, Dartmouth Medical School, Lebanon, NH.
The authors thank Brian Greenlee and Kaloyan Tanev, Neuropsychiatry Fellows James Ford and Heather Pixley, Department of Psychiatry Robert Ferranti, Alice Davison, Shreve Soule, and Robert Shaffer, for their assistance on this project.
This research was supported in part by NIDRR Grants H133G70031 and H133000136, and NIH Grant R01 NS40472-01.