Between 30% and 50% of individuals with HIV exhibit deficits in attention/working memory, motor abilities, episodic memory, and executive functioning,
1–5 which are often attributed to disruptions in frontal-striatal circuitry,
2,6–8 although other patterns of neuropathology also have been found.
9,10 With the advent of highly-active antiretroviral therapy (HAART) in the mid-to-late ′90s,
11 the neurocognitive sequelae of HIV have lessened in severity
12–14 and become somewhat more variable in expression,
15 although mild neurocognitive deficits persist.
13,16–19 Although the current cognitive impairment-profile of HIV is relatively mild for many individuals, it is nonetheless predictive of significant decline in daily functioning.
20 Also, significant variability in HAART adherence patterns among HIV+ individuals
21 may account for recent reports of variable neurocognitive expression of HIV.
15HIV-associated verbal memory impairment is one of the deficits that have persisted despite the advent of HAART. It is not entirely clear how antiretroviral adherence is related to verbal memory abilities in HIV-infected individuals. Pre-HAART research suggested a significant association between memory impairment and lower CD4 T-lymphocyte (CD4) counts,
22 although more recent data from our group suggests that HAART may not be completely protective against memory decline.
23 Moreover, the data on the profile of HIV-related verbal memory impairment are mixed, with some evidence of a primary encoding deficit
24–26 and other evidence of both retrieval and encoding deficits.
27–29 One explanation for this difference is that studies finding retrieval deficits (in addition to encoding deficits) were either conducted before the advent of HAART
27,28 or with samples evidencing relatively low rates of HAART use,
29 whereas those mainly implicating encoding deficits were conducted after HAART use was widespread.
24–26 However, another possibility is that the discrepant findings were due to methodological differences between the studies. More recent studies that support the encoding-deficit hypothesis were based on the examination of list-learning characteristics (semantic clustering,
24 serial-position effects
25,26) and suggested that HIV-associated verbal memory impairment resulted from strategic processing-deficits at encoding; similar deficits have been found in the investigation of working memory in HIV+ participants.
30 The studies finding retrieval-deficits did so primarily by showing disproportionate memory performance benefits with recognition cueing (i.e., better recognition than recall
27,28). This method assumes that greater recognition than recall is suggestive of partial retrieval-deficits.
31 However, recall/recognition discrepancies have been shown to be an imprecise indicator of retrieval ability.
32,33 These methodological difficulties and discrepant findings likely stem from differences in the underlying processes in recall and recognition, with recall being the product of recollection, and recognition resulting from both recollection and familiarity.
34 That said, the findings with recall/recognition discrepancies in HIV+ participants have been fairly consistent and are likely reflective of some degree of retrieval-deficit.
Also, as in other areas of memory research (e.g., experimental amnesia
35), studies of HIV-related memory impairment have varied significantly in the operational definitions and semantic labels used for various memory-process deficits. To reduce any potential confusion in this regard, in the present study and discussion, we used a three-stage/process model of episodic memory:
36 the first process being encoding, where information is taken in and transformed into a format that can be stored in the brain; the second stage is consolidation, where the transformed information is stored in the brain for later use; the third is retrieval, or extraction of the stored information for use.
In the current study, we collected verbal memory and medication-adherence data to determine the relationship between antiretroviral adherence and HIV-related memory impairment. On the basis of previous research, we hypothesized that: 1) poor adherers would demonstrate greater verbal memory impairment than good adherers and HIV-controls; 2) poor adherers would evidence both encoding and retrieval deficits, as compared with controls, whereas good adherers would only show encoding deficits; and 3) the differential memory-deficit patterns of the adherence groups would uniquely contribute to their delayed-recall performances; both encoding and retrieval deficits would account for poor adherers' delayed recall, whereas only encoding deficits would account for good adherers' delayed recall.
METHOD
Participants and Procedures
After providing voluntary written informed consent, participants completed interviews, questionnaires, and a battery of neuropsychological tests as part of a larger project investigating the association between antiretroviral adherence and psychological factors. Trained psychometrists administered the neuropsychological tests and other procedures under the supervision of a board-certified neuropsychologist (CHH). Participants received instructions on how to use Medication Event Monitoring System (MEMS) caps and were scheduled to return 4 weeks later. At the follow-up visit, MEMS caps were collected, and adherence data were downloaded. Participants received $80 for participating in the project, which was approved by the Institutional Review Boards of the University of California, Los Angeles, and the West Los Angeles VA Medical Center.
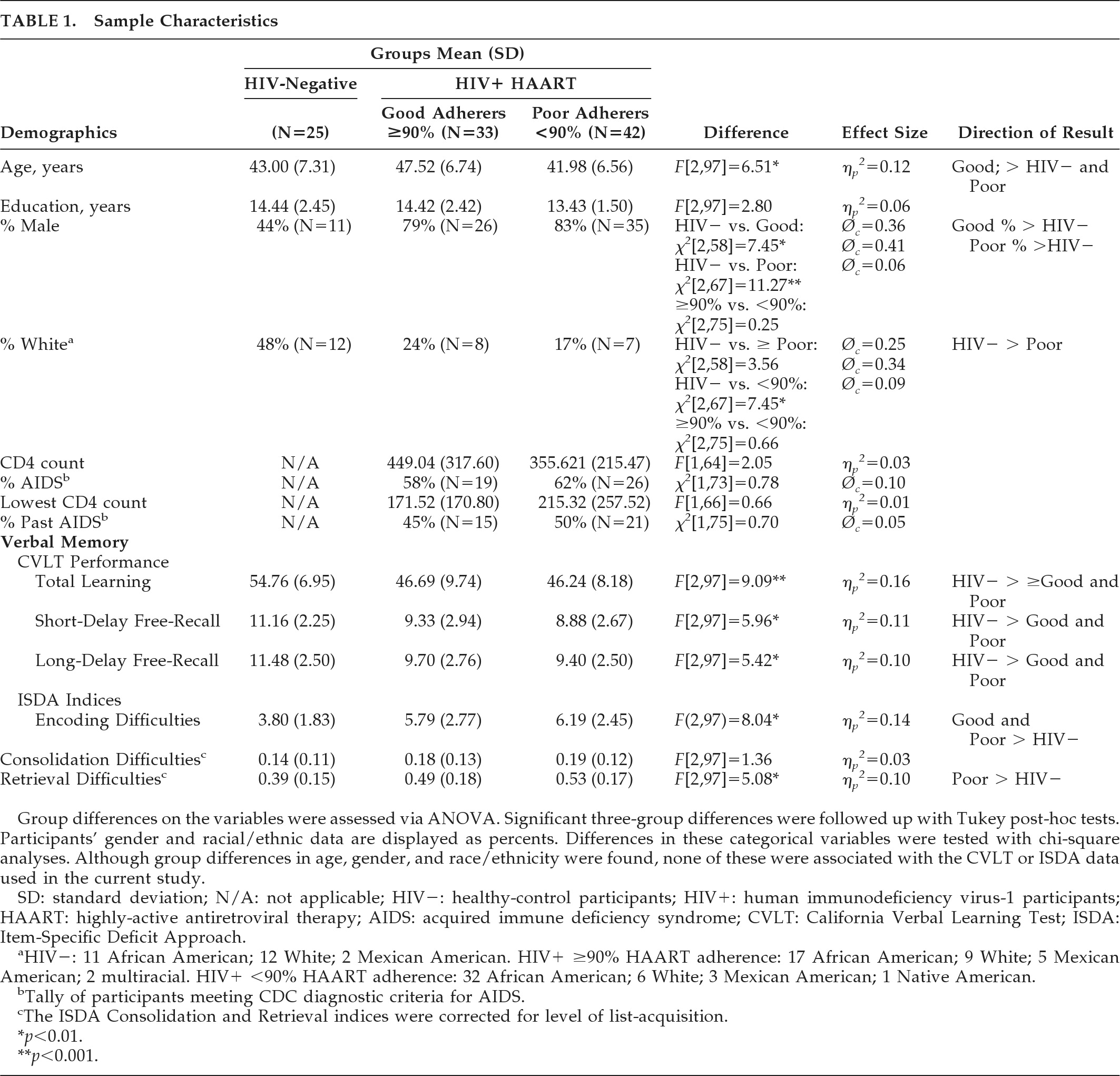
The current study includes MEMS cap and California Verbal Learning Test (CVLT) data from 75 HIV+ participants (33 good antiretroviral adherers and 42 poor antiretroviral adherers) and 25 HIV-negative controls. HIV status was confirmed with ELISA and Western blot; 60% (N=45/75) of the HIV+ participants met the Centers for Disease Control diagnostic criteria for AIDS. All of the HIV+ participants were on self-administered HAART at the time of testing. Exclusion criteria included current substance abuse or dependence, psychotic-spectrum disorders, and other psychiatric disorders (e.g., mood disorders), and history of neurological disorder (e.g., CNS opportunistic infections, traumatic brain injury, stroke). Statistical analyses revealed significant group differences in age, gender, and race/ethnicity among the groups (
Table 1). Specifically, the good-adherence group was older than the other two groups; both HIV+ adherence groups comprised more male participants than the control group; and the poor-adherence group contained more ethnic minorities than the control group. Despite group differences in age, gender, and race/ethnicity, none of these demographic factors were significantly associated with any of the dependent variables in the current study; this result may have been due to restricted ranges in some of these demographic factors. Also, with regard to age, although the differences were statistically significant, they were not necessarily meaningful; all of the participant groups were in the same age-group (middle age). Furthermore, the HIV+ groups were similar with respect to their CVLT performances. Also, regarding the ethnic minority difference, educational quality differences tend to drive lower ethnic-minority performance on neuropsychological tests,
37 which likely reduce as individuals gain access to collegiate educational experiences. The majority of our participants had some college education, which likely reduced the association between minority status and CVLT performances. No other demographic differences were observed. The two antiretroviral-adherence groups were similar in terms of current and lowest CD4 counts and current and past AIDS diagnoses (
Table 1).
Materials and Procedure
Verbal Memory
The CVLT
38 is a standardized verbal list-learning test comprising 16 items that can be grouped into four semantic categories. The list is presented orally to participants over five learning trials. Subsequently, a distractor list is presented, and participants are asked to recall the distractor items. After the distractor trial, participants are administered a short-delay free-recall test, a short-delay cued-recall test, a long-delay (20-minute) free-recall test, a long-delay cued-recall test, and a recognition trial.
Consistent with our operational definitions of encoding, consolidation, and retrieval, the Item-Specific Deficit Approach (ISDA)
32 was applied to the CVLT data to derive indices of encoding, consolidation, and retrieval deficits. The ISDA Encoding Index reflects low acquisition across learning trials. Items recalled less than three times across the five learning trials are summed (maximum value: 16); greater values represent greater encoding difficulties. The ISDA Consolidation Index is calculated by summing the individual items that are recalled during list-learning but not recalled on any subsequent cued- or free-recall trial. The ISDA Retrieval Index is calculated by summing the individual items that are recalled during list-learning but inconsistently recalled across delayed-recall trials (i.e., recalled between one and three times over the four delayed-recall trials). Both the consolidation and retrieval indices are controlled for level of list-learning by dividing by the sum of the individual items recalled during list-learning.
Antiretroviral Adherence
MEMS caps were utilized to track antiretroviral adherence over a 4-week period. MEMS caps use a pressure-activated microprocessor that automatically records the date, time, and duration of bottle-opening. Adherence data were retrieved from each cap via a communication module connected to a PC serial port. For the majority of subjects (61%), MEMS caps were used to track adherence to protease-inhibitors. For those participants on a protease-sparing regimen, MEMS caps were used to track adherence to another antiretroviral medication (e.g., nucleoside reverse-transcriptase inhibitors or non-nucleoside reverse transcriptase-inhibitors). Of our 75 HIV+ participants, 44% (N=33) displayed good adherence, as their MEMS cap data indicated that they were at least 90% compliant with their antiretroviral regimen (Good Adherence Group: mean: 97.33%, SD: 2.68; median: 98.20%; interquartile ratio (IQR): 4.5; Poor Adherence Group: mean: 65.81%, SD: 22.74; median: 73.90%; IQR: 33.13). Previous research suggests that adherence rates of less than 90% lead to increased viral replication and development of drug-resistant HIV strains.
39,40Data Analysis
A significance level of α<0.05 was adopted, unless otherwise stated. We evaluated group differences in demographics and memory performances with either univariate analyses of variance (ANOVAs) or chi-square analyses. Although age, gender, and racial/ethnic differences were found between our groups, these factors were not significantly associated with the CVLT variables used in the current study (r ≤ –0.13, r
pb ≤0.18, r
pb ≤0.10, respectively) or ISDA (r ≤0.14, r
pb ≤ –0.18, r
pb ≤ –0.14, respectively); this result may have been due to restricted ranges in some of these demographic factors. Also, although we found statistically significant age differences, these were not necessarily meaningful; all three groups were in the same age-group (middle age), and both of the HIV+ groups performed similarly on the CVLT. With regard to the minority difference, disparities in educational quality appear to account for a large portion of poorer ethnic-minority performances on neuropsychological tests.
37 These differences likely reduce as minority individuals gain access to collegiate educational experiences. The majority of our participants had completed some college, which may have reduced the association between minority status and CVLT performances. That said, the following analyses were not corrected for age, gender, or race/ethnicity. When three groups were compared by ANOVA (controls versus good antiretroviral adherers versus poor antiretroviral adherers), significant effects were followed up with Tukey's tests. After the determination of verbal memory impairment and specific memory-process deficits, we used hierarchical regression to determine the impact of memory deficits on delayed free-recall of both adherence groups. The predictors were entered into the model based on their temporal relationship with each other (i.e., 1: encoding; 2: consolidation; and 3: retrieval).
RESULTS
Verbal Memory Performances
Univariate ANOVAs demonstrated group differences in total list-learning performance, short-delay free-recall, and long-delay free-recall (
Table 1). Tukey's tests indicated that both HIV+ adherence groups performed more poorly on total recall/list-learning, short-delay free-recall, and long-delay free-recall than the control participants. No significant recall differences were found between the HIV+ adherence groups.
As can be seen in
Table 1, univariate ANOVAs demonstrated group differences in encoding and acquisition-adjusted retrieval deficits, but not consolidation difficulties. Tukey's tests indicated that both adherence groups evidenced greater encoding difficulties than the control participants, and the poor antiretroviral-adherence group showed greater retrieval deficits than the control participants. No significant differences were found between the two adherence groups.
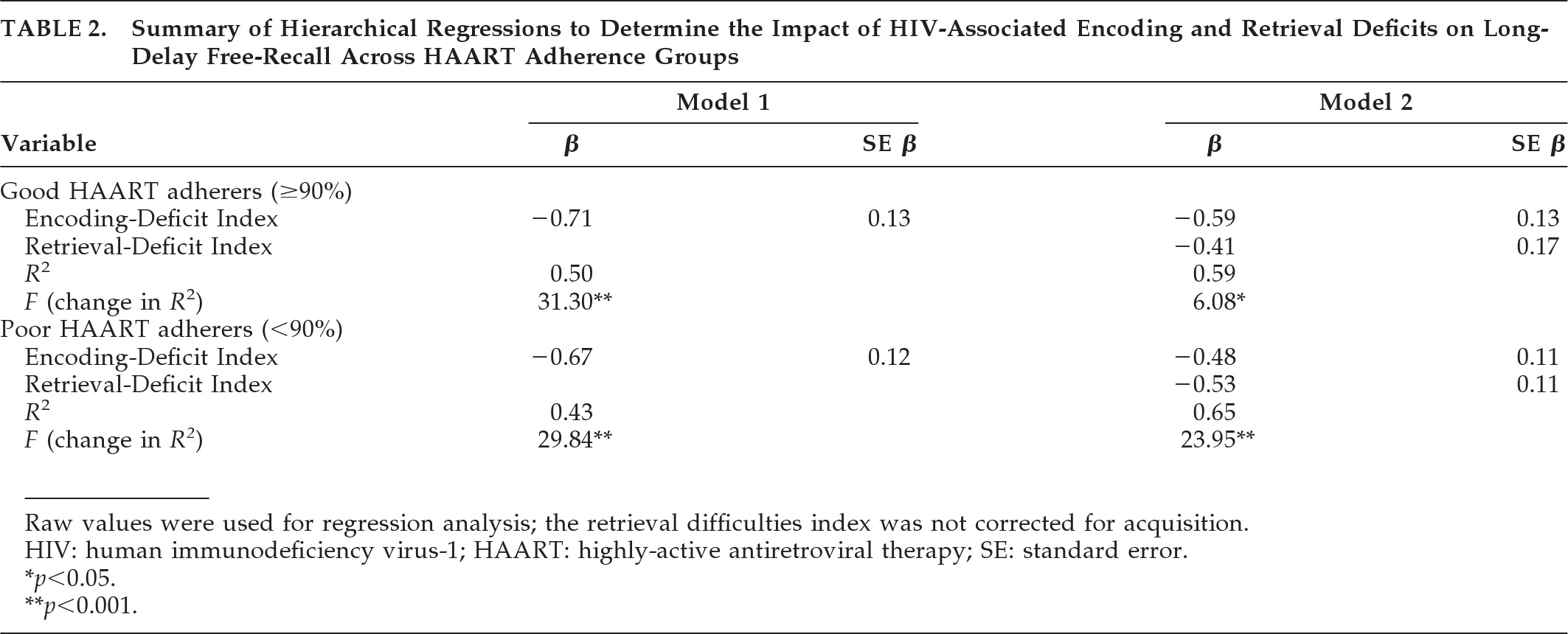
Finally, to determine the impact of encoding and retrieval deficits on the delayed-recall abilities of the two adherence groups, we conducted two hierarchical regressions while excluding uninfected control subjects. Acquisition-corrected values were not used in the regression analyses, since no group comparisons were directly made. As shown in
Table 2, both models were significant and accounted for around 60% of the variance in HIV+ participants' long-delay free-recall. Encoding deficits accounted for most of the variance in both models (50% for good antiretroviral adherers; 43% for poor antiretroviral adherers). Retrieval deficits accounted for a greater degree of additional variance for the poor adherers (22%) in contrast to good adherers (9%).
DISCUSSION
On the basis of previous research, we hypothesized that HIV-infected participants with poor (<90%) antiretroviral adherence would exhibit greater verbal memory impairment, as compared with participants with good (≥90%) antiretroviral adherence and healthy controls. Partial support for this hypothesis was found, as both HIV+ groups demonstrated poorer learning and recall on the CVLT than controls; the two HIV+ antiretroviral-adherence groups did not differ with respect to learning and recall performances. This finding is consistent with data showing verbal learning and recall deficits in HIV+ participants,
2–5,27–29 although it is somewhat inconsistent with data suggesting that HIV-associated neurocognitive deficits might lessen with the use of HAART.
12–14Despite our not finding differences in verbal memory performance between the antiretroviral-adherence groups, we did find a difference in the nature of their verbal memory impairments. As hypothesized, the ISDA, as applied to the CVLT, revealed that poor antiretroviral adherers evidenced both encoding and retrieval deficits, as compared with controls, whereas good adherers showed only encoding deficits as compared with controls. On this basis, some might conjecture that differences in the literature regarding the characterization of HIV-related memory impairment may have been due to disparities in treatment regimens between studies. Specifically, many pre-HAART studies demonstrated both retrieval and encoding deficits
27–29 in HIV+ participants, whereas post-HAART studies indicated that HIV+ participants primarily suffered from an encoding deficit.
24–26 However, it is equally possible that the profile of HIV-related memory deficits is somewhat heterogeneous and that those with more pronounced retrieval deficits have greater difficulties with medication adherence.
Finally, we determined the impact of encoding and retrieval deficits on the delayed free-recall abilities of both adherence groups. We found that encoding was a robust predictor of long-delay free-recall for both good adherers (R2=0.50) and poor adherers (R2=0.43). Also, retrieval-deficits accounted for only a marginal amount of additional variance in the delayed free-recall of good adherers (R2=0.09), but it was a meaningful predictor of long-delay free-recall for the poor adherers (R2=0.22). These results provide good support for our hypothesis that both encoding and retrieval deficits would account for poor-antiretroviral adherers' delayed recall, whereas only encoding deficits would account for good-adherers' delayed recall.
It is not currently clear how HAART might affect cognition, although more recent data suggest that it is associated with neuropsychological improvement at 12-to-48 months post-initiation, particularly for HAART regimens with greater CNS-penetration.
41 It is possible that good antiretroviral adherence may lead to greater reduction of HIV replication in the brain, and particularly in the striatum. Such an effect could account for our results, as the striatum has been shown to play a unique, although not completely direct, role in memory retrieval.
42,43 That said, our data cannot address the possible causal relationship between HAART adherence and HIV-associated memory impairment. The potential neural mechanism(s) by which HAART might improve or protect memory is currently unknown and remains a topic for future research.
In sum, we found convincing evidence that HIV is associated with encoding and retrieval deficits for verbal material, via application of the ISDA to CVLT performances; it should be noted that this study represents that first published application of the ISDA to memory impairment in HIV. We found that good antiretroviral adherers showed only encoding deficits, whereas poor antiretroviral-adherers demonstrated both encoding and retrieval deficits. It should be noted that the disparities found between our antiretroviral adherence groups cannot be attributed to differences in current or past CD4 counts or current or past AIDS diagnoses. Given the cross-sectional nature of our study, we cannot conclude that suboptimal adherence results in a change in verbal memory, although our findings, in concert with other research
12–14,41 on the relationship between cognition and antiretroviral use, suggest this may be the case. However, a competing and equally viable explanation is that preexisting HIV-related retrieval deficits in some individuals may reduce their ability to adhere to medications. Indeed, research has shown that memory is predictive of antiretroviral adherence in individuals with HIV.
23 Longitudinal studies will be necessary to clarify the relationship between antiretroviral adherence and impairments in verbal memory and other cognitive abilities affected by HIV. Also, our antiretroviral-adherence groups were well matched on indicators of HIV severity (CD4, AIDS diagnoses), which may have reduced our ability to discern additional verbal memory differences and possible interactions between levels of antiretroviral adherence and disease severity on verbal memory abilities. Future work with larger sample sizes will be needed to determine any possible dose effects of antiretroviral medications on cognition at different HIV severity levels. Still, our findings highlight the complex relationship between antiretroviral adherence and the nature of HIV-related verbal memory impairment. Moreover, our data also suggest that although HIV+ individuals with good antiretroviral adherence may benefit from the use of memory-encoding strategies to improve their recall of new information, those individuals who also have poor antiretroviral adherence will likely need both encoding and retrieval aids. Although we are unaware of any studies examining the impact of neurocognitive rehabilitation for HIV-related memory impairment, it may be useful to keep the current study in mind when working with HIV/AIDS patients. Having patients repeat and elaborate to-be-learned information (e.g., new appointment schedule, medication changes) may help them better encode and retain that material, and providing them with retrieval cues (e.g., reminder calls, timed reminders from electronic organizers), particularly for those with poorer HAART adherence, may improve their later recall of said material.
Acknowledgments
A portion of this work was presented at the 21st Annual Meeting of the American Neuropsychiatric Association in Tampa, FL.
Location of Work: Department of Psychology, VA Greater Los Angeles Healthcare System, West Los Angeles Healthcare Center, Los Angeles, CA.
Funding provided by NIMH (NIMH) RO1 MH58552 to Dr. Hinkin. Dr. Wright was supported by NIMH Institutional Training Grant T-32 MH19535.