The discovery of a novel protein and its interaction with a serotonin receptor on the surface of neuronal cells could prove to validate the fundamental biological basis of mood disorders, particularly depression.
The groundbreaking identification of the new protein, called p11, was reported in the January 6 Science by Nobel Laureate Paul Greengard, Ph.D., a professor of psychiatry and pharmacology and director of the Laboratory of Molecular and Cellular Neuroscience at Rockefeller University.
Greengard shared the 2000 Nobel Prize in Physiology or Medicine with psychiatrist Eric Kandel, M.D., of Columbia University and Arvid Carlson, M.D., of the University of Goteborg in Sweden (Psychiatric News, November 3, 2000). Greengard received the Nobel for his work in defining the interplay of proteins and hormones with receptors on the cell surface. It was Greengard's work that defined the mechanism by which extracellular proteins cause an intracellular effect without having to enter the cell itself.
In 2002 Greengard again made headlines with his lab's determination of the cellular interactions of the antidepressant fluoxetine. At that time, Greengard and his colleagues detailed the interaction between fluoxetine and a cell surface protein known as DARPP-32 (Psychiatric News, May 17, 2002).
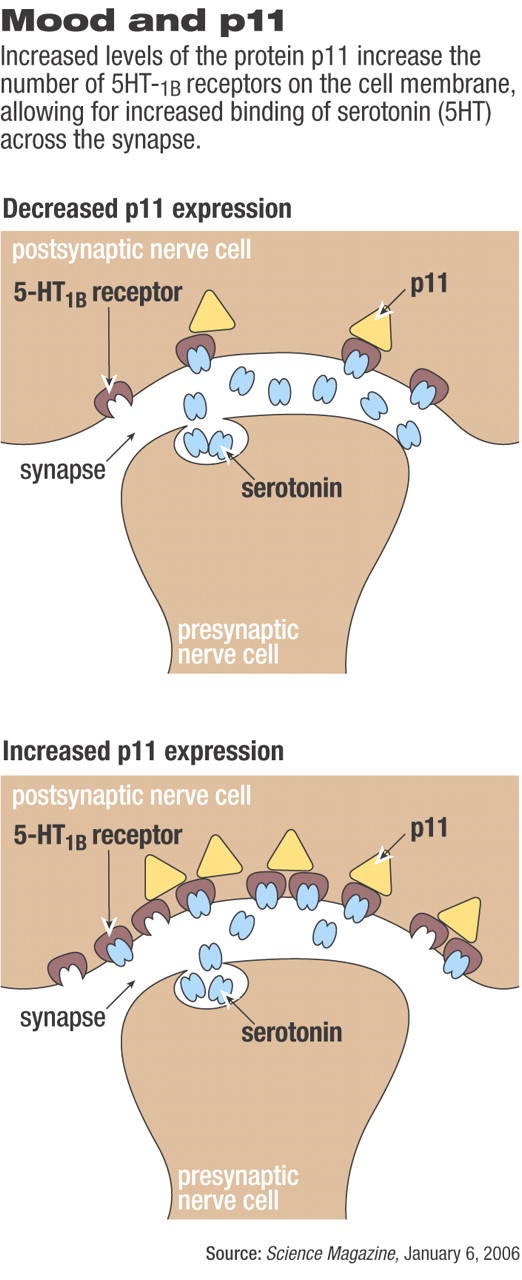
Greengard's latest contribution to advancing the understanding of the cellular mechanisms underlying depression involves the serotonin 1b receptor (5-HT1B) and its interaction with the extracellular protein called p11. Simply put, when p11 levels increase, the number of 5-HT1B receptors on the cell surface increases proportionately. With more 5-HT1B receptors on the surface of the neuron, serotonin communication across the synapse is made more sensitive and more effective. However, when p11 levels are low, fewer 5-HT1B receptors migrate from inside the neuron to the cell membrane at the synaptic clef, leading to decreased efficiency and power of serotonin signaling.
To explore how the 5-HT1B receptor functioned, Greengard and his colleagues conducted laboratory tests to determine with what proteins the receptors interacted. They found the interaction with p11 and hypothesized that p11 played a role in recruiting serotonin receptors to the cell surface, where they are functional.
Greengard and his colleagues suspected that p11 levels might be directly involved in the development of depression, anxiety, and similar psychiatric illnesses thought to involve faulty serotonin receptors. The researchers next examined p11 levels in postmortem samples from the brains of depressed patients and a mice model of depression (called “helpless mice”) and compared the levels with those found in non-depressed humans and normal mice. Levels of p11 were found to be substantially lower in depressed humans and the “helpless” mice.
“Mice deficient in this protein—p11—sidebar depressionlike behaviors, while those with sufficient amounts behave as if they have been treated with antidepressants,” Greengard said in a prepared statement.
“This new-found protein,” added Elias Zerhouni, M.D., director of the National Institutes of Health (which funded Greengard's work through grants from the National Institute of Mental Health), “may provide a more specific target for new treatments for depression, anxiety disorders, and other psychiatric conditions thought to involve malfunctions in the serotonin system.”
Greengard's team next examined the effect of treatments that are used in humans and designed to boost weak serotonin systems on p11 levels in brain cells by administering two types of antidepressants—one tricyclic, the other a monoamine oxidase inhibitor. They also tested the effect of electroconvulsive stimulation in rats.
“These three different ways of treating depression all caused an increase in the amount of p11 in the brains of these mice,” Greengard said. “They work in totally different ways, but in all cases they caused the same biochemical change. So, it is pretty convincing that p11 is associated with the main therapeutic action of antidepressant drugs.”
The researchers then altered the expression of the gene coding for p11 in mice. As they hypothesized, mice with increased expression of the p11 gene acted “less depressed.” In addition, on examination of brain tissue from these mice, the researchers saw increased levels of 5-HT1B receptors at the cell surface.
The researchers reached the opposite conclusion when they molecularly knocked out the p11 gene in mice. Compared with control mice, the knockout mice had fewer 5-HT1B receptors at the cell surface, had reduced levels of serotonin signaling, had decreased responsiveness to sweet rewards, and were less active (all signs considered to be “depressionlike” in mice).
In an accompanying editorial, Trevor Sharp, M.D., an Oxford University pharmacologist, wrote, “Overall, [these findings] represent compelling evidence that p11 has a pivotal role in both the cause of depression and perhaps its successful treatment.” Sharp noted that depression and the relief of its symptoms “are likely to be influenced by many different genes,” and the current report adds to the list, “not just through the addition of p11, but also the large number of serotonin receptor-interacting proteins that p11 represents.”
Sharp concluded, “The case for p11 as a key molecule in mood regulation is convincing, and it is now timely for translational science to take this exciting development to the next step.”
An abstract of “Alterations in 5-HT1B Receptor Function by p11 in Depression-Like States” is posted at<www.sciencemag.org/cgi/content/abstract/311/5757/77>.▪
Science 2006 311 77