Depression in childhood and adolescence is frequently underdiagnosed and undertreated (
1 ). Selective serotonin reuptake inhibitors (SSRIs) are a promising drug class for treating children with depression, and trials have demonstrated a benefit of treatment with fluoxetine (
2,
3 ). However, reviews of published and unpublished data for SSRIs—including paroxetine, citalopram, and sertraline—have suggested an increased rate of suicidality or suicidal ideation among children treated with SSRIs compared with those treated with a placebo (
4,
5,
6,
7,
8,
9 ). Warnings were issued by regulatory agencies in the United Kingdom in late 2003 (
10 ) and by the U.S. Food and Drug Administration during the following year (
11 ). These clinical issues, combined with the difficulty of treating depression among children, leave clinicians in a conundrum. Further complicating these treatment decisions are economic concerns, as many antidepressants are very expensive, although some have recently become available in generic forms.
Medicaid, the joint state and federal health insurance program for low-income Americans, is an important provider of coverage for mental health care (
12 ). Children covered by Medicaid use a considerably higher amount of mental health services than children with private insurance (
13 ). Although payments to providers account for much of the cost of mental health services, coverage of medications is another important issue, especially for state Medicaid programs facing budget constraints. In response to increasing medication costs, many state Medicaid programs have implemented prior-authorization policies for high-cost drugs, requiring that physicians provide clinical justification before prescriptions will be covered (
14 ).
We studied state Medicaid programs to determine the frequency of prior-authorization requirements for antidepressant medications for children.
Methods
We contacted Medicaid administrators by e-mail and telephone for details of specific policies concerning antidepressants for children. Data collection occurred between June and August 2005. Specific data items included whether any of the antidepressant drugs required prior authorization (for all patients or for children alone), the date of policy implementation, and a description of the authorization process. Additional information was collected from the Web site of each Medicaid program.
When prior-authorization programs were present, we checked which SSRIs were restricted. At the time of our survey, the largest published trial involving children suggested that fluoxetine has beneficial effects among children (
2 ), and generic fluoxetine represented one of the least expensive SSRI options, so we determined whether policies promoted the use of fluoxetine. Additional data were recorded about whether the policy differed across age groups of children and whether the prescribing of SSRIs for children was restricted to psychiatrists or pediatricians. We tabulated how frequently fluoxetine could be prescribed without restriction, whether any of the other SSRIs could be prescribed without restriction or required prior authorization, whether older medications such as tricyclic antidepressants could be prescribed without restrictions, and whether pediatric patients already on a given antidepressant would be able to continue it without a new prior authorization.
Results
Data were available from 49 states and the District of Columbia. Data for Arizona were not available because it administers its Medicaid program through a set of subcontracts and does not have statewide drug policies (
15 ). Thirty states (60%) had prior-authorization requirements for some antidepressant medications. Of these 30 states, 22 (73%) had no special provisions for children, so that restrictions for SSRI prescribing for children were the same as those for adults. The other eight (27%) of these states had specific regulations regarding children.
Among the eight states with prior-authorization provisions for children, most dealt with specific drugs. Two state programs had a special provision for sertraline, requiring prior authorization for adult patients but not for patients aged six to 17 years. Another state had paroxetine available without prior authorization for most patients, although it required approval for patients younger than 18 years. Two states included provisions for specific non-SSRI medications (amoxapine, imipramine, and a fluoxetine and olanzapine combination), and another state exempted all patients younger than 12 years from its prior-authorization process for antidepressants.
The remaining two states had more detailed policies for children. Maine included a strong warning for use of SSRIs among pediatric patients, requiring that new starters of antidepressant therapy try fluoxetine before another antidepressant is authorized. Established users of other antidepressants are "grandfathered" out of the prior-authorization process. Delaware's program was the most detailed, including a requirement for an evaluation by a psychiatrist or mental health provider and documentation of behavioral evaluation for dosage changes. Policies did not distinguish between whether the SSRIs were prescribed for depression or for anxiety disorders.
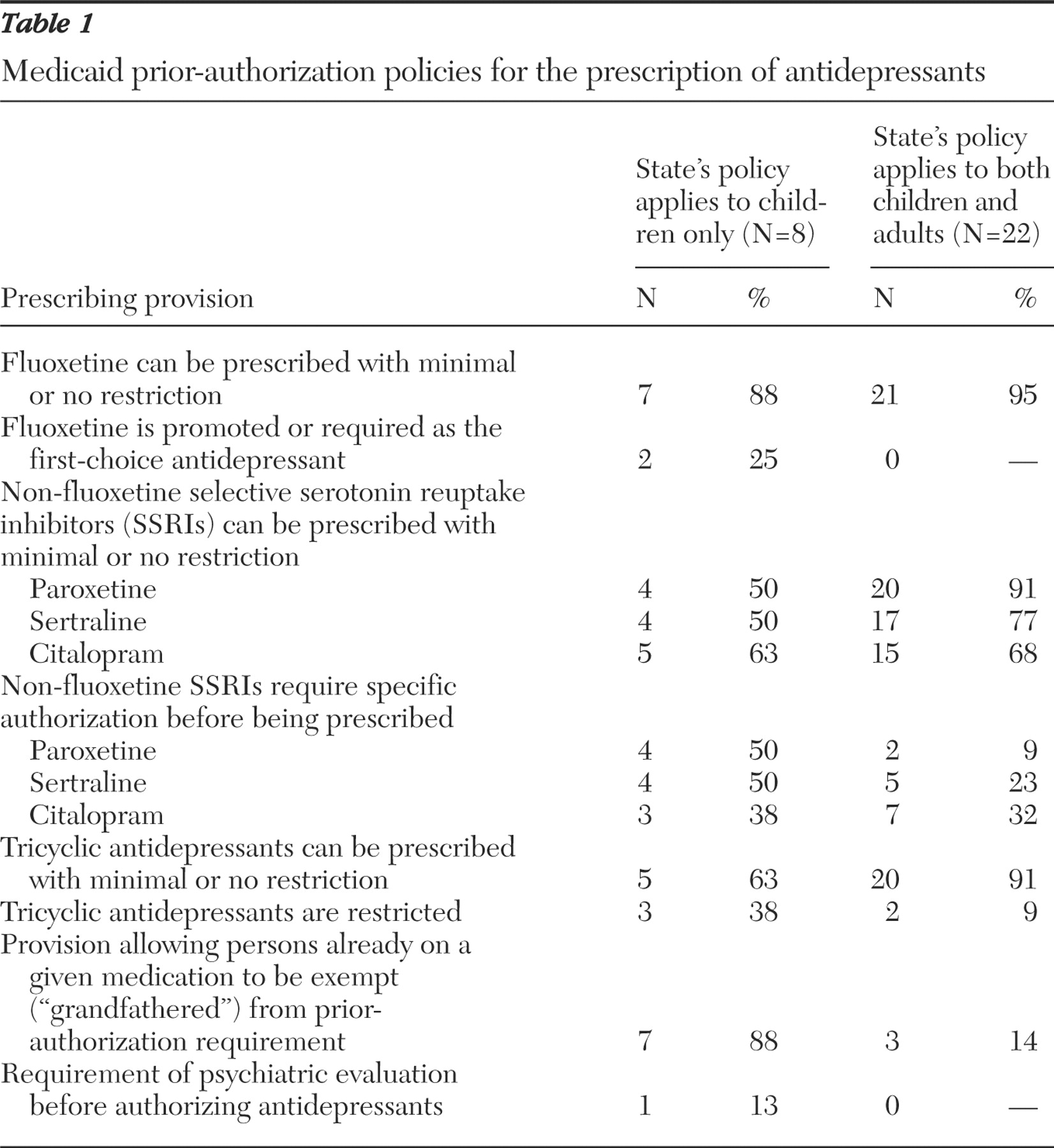
Table 1 summarizes the overall stance of prior-authorization policies on prescribing antidepressants to children. The first column summarizes provisions in the eight states with policies specifically directed toward children, and the second column describes general restrictions created by prior authorization in the other 22 states with such policies, restrictions that affect adults and children equally. The remaining 20 Medicaid programs did not have policies regarding antidepressants. In all but two states, fluoxetine could be prescribed for children without restriction; this includes states with no prior-authorization program and states with no special provision for children. In one state with a general prior-authorization program (no mention of children), other SSRIs were preferred and fluoxetine required prior authorization. Delaware's requirement of psychiatrist evaluation represents the only other hurdle.
Fluoxetine was the stated first-choice agent for children in two states and was easily available in most states with a prior-authorization program for antidepressants for all ages, even when it was not specifically promoted as the first choice. In most states, at least some of the non-fluoxetine SSRIs could be prescribed for children without prior authorization; paroxetine and sertraline were restricted in four of the eight states with prior authorization specific to children and citalopram was restricted in five of those states. In a majority of states with general prior-authorization policies for antidepressants, those three agents could be prescribed with limited restriction. Three states specifically restricted tricyclic antidepressant use among children, and two of the 22 states with general prior-authorization policies placed a barrier on tricyclic antidepressant use for all patients. Seven states included a "grandfather" provision for children that would allow continuation of a previously started SSRI, although many of these approvals would only be in effect for the first six months after the implementation of prior authorization.
Discussion
Prior authorization has been used with increasing frequency to control medication use in public and private health insurance plans. Prior-authorization requirements have generally been implemented as cost-control measures, often accompanied by other strategies, such as increased patient cost-sharing. Included in these policies is usually recognition that some medications need to be used in selected clinical circumstances despite their cost. In this way, prior-authorization programs can directly affect the quality of medical care—that is, well-designed policies may promote high-quality and cost-effective practice, whereas poorly conceived policies may result in suboptimal prescribing and eventual adverse outcomes. Medicaid coverage of antidepressants for children provides an important case study of prior authorization application: it involves a disease associated with significant medical and social costs, a choice among several agents of varying costs, and payers with constrained budgets. The heterogeneity that we observed in state policies, without clear indications that clinical evidence is incorporated into the development of a prior-authorization policy, represents a notable shortcoming.
Many state Medicaid programs put no restriction on reimbursement for antidepressants. In these situations, payment is provided as it would be for any other medication and prescribing quality and safety are the responsibility of the physician writing the prescription and the pharmacist filling it. When prior authorization is required, patients can still fill the prescription out-of-pocket, but for most Medicaid beneficiaries paying retail price for these medications is not a realistic option. In these circumstances, the patient or pharmacist will need to contact the prescribing physician in order to obtain an alternative prescription or initiate an authorization process. Prescribing a preferred medication will represent the lowest administrative burden for physicians and is likely to be an appealing option. How often such switches are made, and their clinical consequences, is not known and would be a fruitful subject for future research.
In 30 states with Medicaid prior-authorization policies for antidepressants, only eight include specific components that are relevant for children. The policies in these eight states vary considerably and do not appear to reflect recent concerns about suicidality associated with SSRI use. Two states use Medicaid prior authorization as a tool to push children toward first-line use of fluoxetine, perhaps reflecting the clinical evidence that was current at the time of policy development. Future research focusing on state characteristics as predictors of prior-authorization policy decisions may provide important additional insights into policy development.
Our study had several limitations. State policies may have changed since our initial data collection, and our results would not include those changes. In addition, some states may have had policy requirements that were not captured in our review or were unwritten. Actual policy implementation may have deviated from the requirements listed in the written policies. Our data collection did not reveal at what level of detail authorization requests were reviewed by state Medicaid programs before payment would be allowed. We suspect that rejected authorization requests can be appealed by patients, pharmacists, or physicians, but descriptions of such processes were not provided in the information that we received. This general review does not capture the subtleties of psychiatric prescribing for children nor does it describe all elements of the current controversies over antidepressants and suicide.
Even with the limitations described above, these observations have important implications for the development of rational drug reimbursement policy for psychotropic medications. Given the prominence of prescription drug expenditures as a driver of rising health care costs, prior-authorization policies are likely to become much more widespread in the future. Improved design of these policies can contain costs and, when these policies are implemented with clinical justification, they can also lead to better mental health care. Against this backdrop, it is concerning that the small number of policies making specific provisions for children differ so much in the approaches that they adopt. The question of whether insurers face any liability for the impact of reimbursement policy on clinical decisions is not settled but may represent another important motivation for Medicaid, and all insurers, to arrive at the best criteria for drug reimbursement guidelines. An important additional consideration is the potential barriers to care created by these policies, which may require considerable physician and office staff time to obtain authorization.
Conclusions
Patients and the public will be best served when the development process for these policies is transparent and when clinical criteria are included and drawn from the best and most current evidence. Both government insurance programs and private insurers can draw on clinical experts to develop evidence-based recommendations that can guide policy. The Agency for Healthcare Research and Quality has National Evidence-Based Practice Centers that could fill this function, drawing on models such as the National Institute for Clinical Excellence in the United Kingdom. The relationship between the evidence-base for prior-authorization policies and their clinical impact is an important topic for additional research and should inform the development of more rational policies in the future.
Acknowledgments and disclosures
The authors gratefully acknowledge Elizabeth Robinson, B.A., and Hailu Cheng, B.A., for gathering data.
The authors report no competing interests.