The introduction of antipsychotics in depot formulation was primarily aimed at improving medication adherence because better adherence is associated with more favorable outcomes. Adherence is a complex construct that has been measured in various ways, including time to medication discontinuation for any reason, a measure that may reflect a medication's acceptability from patients' and clinicians' perspectives and may provide an index of a medication's efficacy, safety, and tolerability (
1 ).
In the treatment of schizophrenia, meta-analyses of randomized controlled trials of antipsychotics reported more favorable outcomes for persons treated with depot first-generation antipsychotics, compared with those treated with the oral form, (
2,
3 ) but not lower treatment discontinuation rates (
3 ). Although unexpected, that finding may not reflect treatment in real-world settings because randomized controlled trials may minimize differences in outcomes between treatments, likely owing to selection of cooperative patients willing to enter a clinical trial, close clinical supervision, and typically short trial durations. Moreover, in double-blind randomized controlled trials (
4 ), the oral treatment group receives dummy injections in addition to active oral medication, thus introducing an artificial and potentially adherence-enhancing treatment component that is absent in usual care.
Using data from a large, prospective, noninterventional, observational study of treatment for schizophrenia in the United States, this study compared—over a one-year period—the time to medication discontinuation for any reason among persons initiated on first-generation antipsychotics in oral or in depot formulation.
Methods
We used data from a large (N=2,327), nonrandomized, noninterventional, three-year, prospective, multisite study of treatment for schizophrenia (U.S. Schizophrenia Care and Assessment Program) conducted from July 1997 to September 2003. Details about study methods and sample characteristics are available elsewhere (
5 ). In brief, participants were adults, primarily outpatients, and diagnosed as having schizophrenia, schizoaffective, or schizophreniform disorders on the basis of
DSM-IV criteria. Broad inclusion criteria (allowing substance use and other comorbid conditions) aimed to enhance the generalizability of the findings to treatment in usual-care settings. Excluded were individuals unable to provide informed consent and those who had participated in a clinical trial within 30 days of enrollment. Participants were enrolled at six sites in six states and treated in diverse systems of care, including community mental health centers, university health care systems, the Department of Veterans Affairs health services, and community and state hospitals. Institutional review board approvals were received, and informed consent was provided by all participants.
All treatment decisions, including any medication initiations and discontinuations, were made by treating physicians as they occurred in usual care. The analysis presented here included participants who were initiated—at any time during the three-year study—on haloperidol or fluphenazine in oral or depot formulations, were treated with the medication for at least 60 days after initiation, and had medication information for at least one year after initiation.
Systematic abstraction of patients' medical records was performed at baseline and each six-month interval thereafter by trained and annually certified examiners, which provided information about prescribed medications. Psychiatric scales were administered at predetermined intervals but were not set to coincide with time of initiation or discontinuation of any medication.
Consistent with prior research (
5 ), time to all-cause medication discontinuation during the first year after medication initiation was defined as a medication switch or discontinuation as marked by the first medication gap more than 30 days after medication initiation.
Time to all-cause medication discontinuation was assessed by using Kaplan-Meier survival analysis (log-rank test) and a Cox proportional hazards regression model. To control for potential selection bias, the analysis employed propensity score-adjusted bootstrapping resampling methods, with 1,000 reiterations. To help address possible group differences at the time of medication initiation in this nonrandomized study, analyses (excluding survival analysis) were adjusted for a set of variables selected a priori: gender, ethnicity, psychiatric hospitalization in the 60 days before initiation, schizoaffective disorder diagnosis, comorbid substance use diagnosis, number of days of antipsychotic use in the 60 days before initiation, type of depot (fluphenazine, haloperidol), study site, and days from patient enrollment date to date of study start (July 1997). Most of these variables were previously associated with nonadherence in the treatment of schizophrenia (
6 ). To assess robustness of the medication discontinuation definition, we conducted sensitivity analyses, altering the length of the first medication gap to at least twice the depot injection standard frequency based on product insert, resulting in a medication gap of more than 28 days for the fluphenazine depot and more than 60 days for the haloperidol depot. Analyses were also repeated by including participants with any treatment duration after medication initiation. Statistical analyses used SAS, version 8.2. Significance level was set at
α =.05.
Results
Participants treated with depot first-generation antipsychotics (N=97) and oral first-generation antipsychotics (N=202) did not significantly differ on clinical or demographic baseline characteristics—for example, age, gender, ethnicity, insurance type, schizoaffective diagnosis, prior hospitalization, substance use diagnosis, or total scores on the Positive and Negative Syndrome Scale.
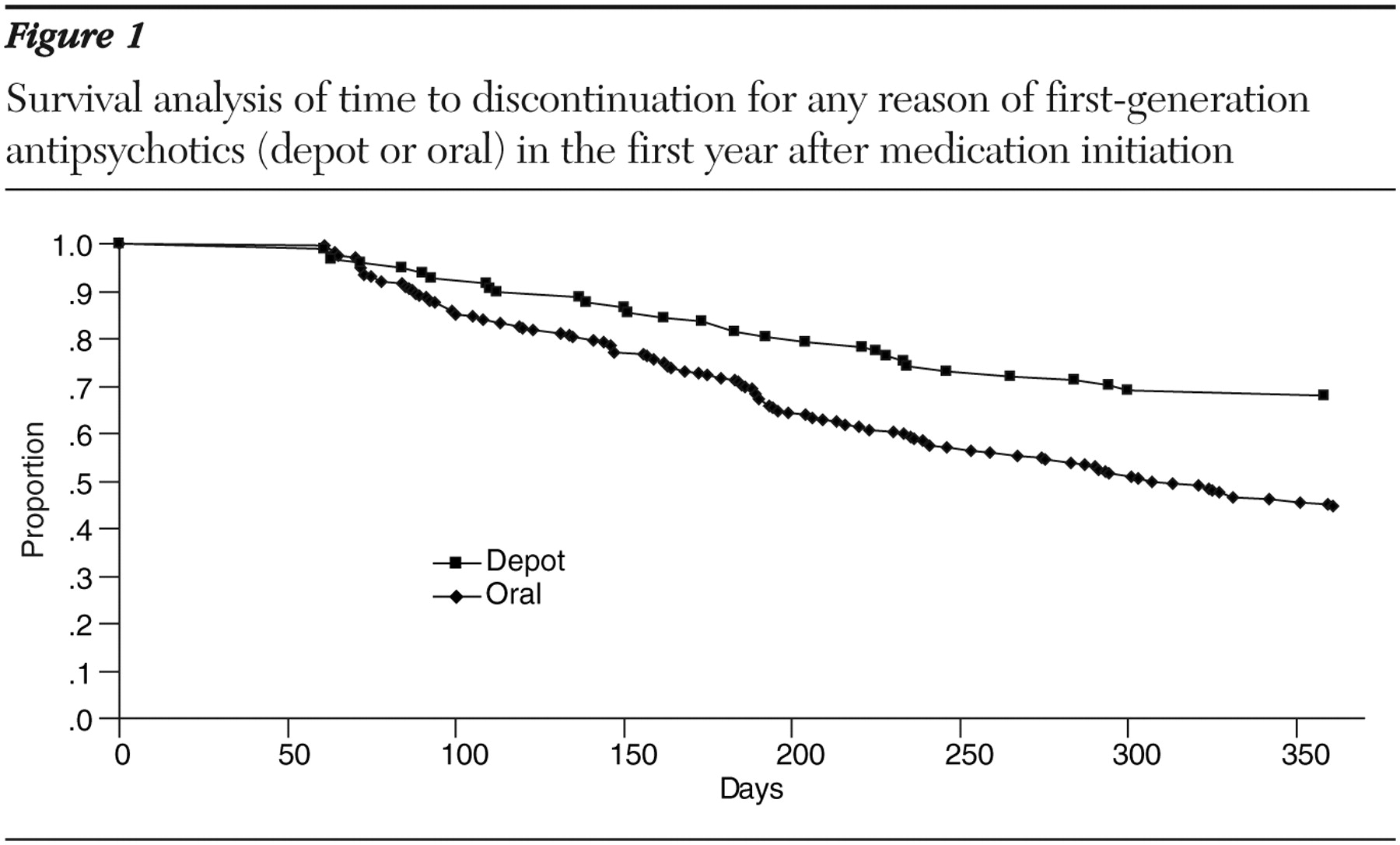
Modal depot dose and frequency was 25 mg per biweekly injection of fluphenazine (N=47) and 100 mg per monthly injection of haloperidol decanoate (N=50). Mean oral doses were 12 mg per day for fluphenazine (N=93) and 10.7 mg per day for haloperidol (N=109). Compared with participants treated with oral antipsychotics, the depot-treated participants had a significantly (p<.01) longer time to discontinuation (fluphenazine: depot, mean±SD of 292±106 days, versus oral, 270±108 days; haloperidol: depot, 316±93 days, versus oral, 257±115 days) and were twice as likely to stay on the medication (log-rank, p<.001; Cox model, p=.002; hazard ratio=1.94, 95% confidence interval=1.3–2.9) (
Figure 1 ). Sensitivity analyses confirmed the findings (all p<.05).
Discussion
In this large observational study of treatment for schizophrenia in usual care, participants who were initiated on depot first-generation antipsychotics had a significantly longer mean time to all-cause medication discontinuation and were twice as likely to stay on the medication for one year, compared with participants treated with the oral formulation. To the best of our knowledge, this is the first study in real-world practice settings to compare treatment duration with depot versus oral formulation of the same antipsychotic medications. Findings are consistent with nonrandomized studies (
7,
8 ) that reported longer time to all-cause discontinuation for depot compared with oral first-generation antipsychotics when comparing drug formulations, not necessarily of the same drugs. Current findings help highlight the adherence advantage of depot over oral antipsychotics in usual clinical practice and extend randomized controlled trial-based research on the benefits of treatment with depot formulations, because longer treatment duration is a major driver of better clinical and functional outcomes in the long-term treatment of patients with schizophrenia (
9 ).
This study has its limitations. First, medication information was based on prescriptions in medical records; thus it may not reflect actual filling of the prescription or receipt of the medication. However, previous research has demonstrated high concordance between prescription and fill of prescription in a similar patient population (
10 ). Moreover, using prescription data may also be the study's strength, because the number of days supplied in paid-claim databases for depot prescriptions was found to be recorded in an inconsistent or inaccurate manner. Second, potential selection bias cannot be ruled out because statistical analyses could adjust only for available preinitiation and baseline characteristics, not for clinical status at the time of medication initiation.
Conclusions
Results from this large, long-term, observational study suggest that in the usual care of persons with schizophrenia, treatment with antipsychotics in depot formulation may provide an adherence advantage over the oral formulation of the same medication, as indicated by longer time to medication discontinuation.
Acknowledgments and disclosures
The US-SCAP study was funded by Eli Lilly and Company and administered by the Medstat Group. The authors thank the site investigators and others who collaborated in the research. Maryland: Anthony F. Lehman, M.D., M.S.P.H., University of Maryland School of Medicine, and Gerard Gallucci, M.D., M.H.S., Johns Hopkins Bayview Medical Center (formerly). Colorado: Courtenay Harding, Ph.D., University of Colorado (formerly). Florida: David Shern, Ph.D., Florida Mental Health Institute, University of South Florida (formerly), and Terri Saunders, M.S. (formerly Florida Mental Health Institute). North Carolina: Jeffrey Swanson, Ph.D., Lawrence A. Dunn, M.D., and Marvin Swartz, M.D., Duke University Medical School. California: Richard L. Hough, Ph.D., and Concepcion Barrio, Ph.D., Child and Adolescent Services Research Center and San Diego State University. Connecticut: Robert A. Rosenheck, M.D., and Rani Desai, Ph.D., Department of Veterans Affairs Connecticut Health Care System. Medstat group: Pat Russo, Ph.D., M.S.W. (formerly), Liisa Palmer, Ph.D., Lito Torres, M.B.A., and Brian Cuffel, Ph.D. (formerly). Eli Lilly and Company: Don Buesching, Ph.D., Brian M. Johnstone, Ph.D., and Thomas Croghan, M.D. (formerly). Consultants: David Salkever, Ph.D. (formerly of Johns Hopkins University), Eric P. Slade, Ph.D. (formerly of Johns Hopkins University), William Hargreaves, Ph.D., and Martha Shumway, Ph.D., University of California, San Francisco.
Eli Lilly and Company sponsored this study. Dr. Zhu, Dr. Ascher-Svanum, Dr. Faries, and Mr. Montgomery are employees and minor stockholders of Eli Lilly and Company. Dr. Shi received a consulting grant and unrestricted research grants from Eli Lilly and Company. Dr. Marder has received grant support from Eli Lilly and Company and Janssen Pharmaceuticals and is a consultant for Bristol-Myers Squibb, Otsuka American Pharmaceuticals, Solvay Pharmaceuticals, and Pfizer.