Medicare Part D refers to a voluntary outpatient prescription drug benefit program implemented on January 1, 2006, for Medicare beneficiaries. However, several categories of medications are excluded from Part D coverage, including benzodiazepines. Health care providers and policy makers raised concerns about benzodiazepines' exclusion from Medicare reimbursement (
1,
2 ) because of the widespread use of these drugs for many common medical conditions, including anxiety and seizure disorders. Indeed, in 2006 a total of 80.1 million benzodiazepine prescriptions were dispensed, making benzodiazepines the tenth most frequently used therapeutic class in the United States (
3 ). A recent report found that more than 12% of nursing home residents dually eligible for Medicare and Medicaid benefits have at least one benzodiazepine prescription filled monthly (
4 ). One prospective study of anxiety disorders (Harvard/Brown Anxiety Research Project) found that benzodiazepines remained the most commonly used class of drugs for panic disorders for the past decade (
5 ).
Benzodiazepines are indicated for treating generalized anxiety, panic attacks, bipolar disorders, insomnia, seizure disorders, and muscle spasms—conditions common in older adults and disabled individuals, who make up the Medicare population (
6,
7,
8 ). All benzodiazepines in the United States are generically available and are therefore relatively inexpensive. Yet despite benzodiazepines' effectiveness and widespread acceptance, their use in the treatment of psychiatric and medical conditions remains controversial (
9 ). High efficacy, low toxicity, and rapid onset of action are the primary advantages of benzodiazepines over alternative medications (for example, barbiturate and nonbarbiturate compounds). In contrast to the delayed anxiolytic effects of other drugs, including buspirone and newer-generation antidepressants, the immediate effect of a single benzodiazepine dose and its comparative low toxicity make benzodiazepines highly effective in treating acute anxiety (
6 ) and arresting status epilepticus for patients with seizure disorders (
10 ). The medical utility of benzodiazepines is globally recognized; diazepam is included in the World Health Organization's 2005 Model List of Essential Medicines as the recommended medicine to treat generalized anxiety, sleep disorders, and seizure disorders (
11 ).
Benzodiazepines also have potential negative side effects. Because of their potential for abuse and dependence, benzodiazepines are not suitable for long-term treatment, especially among vulnerable elders (
12 ). Indeed, several long-acting benzodiazepines are included in the Beers List, a guide that identifies medications to be avoided by the elderly (
13 ). Specifically, benzodiazepine use has been recently associated with increased risk of falls and hip fractures among the elderly (
14 ).
Concerns regarding the safety and inappropriate use of benzodiazepines led to their ultimate exclusion from Part D coverage. Such exclusion from government reimbursement, however, is not new; state Medicaid programs exempted benzodiazepines from federal reimbursement with the passage of the Omnibus Budget Reconciliation Act of 1990 (
1 ). Although all Medicaid programs currently cover benzodiazepines, at least 19 Medicaid programs impose coverage restrictions of some sort (
2 ). Furthermore, many states have included benzodiazepines in their prescription drug monitoring programs (
15 ), which reduce benzodiazepine use in the general population and among patients with severe psychiatric and physical conditions (
15,
16,
17 ). Thus the exclusion of benzodiazepines may affect the physical and mental health of economically disadvantaged elderly persons and beneficiaries with disabilities—those most requiring these medications. Without drug coverage, those who are currently clinically stabilized on benzodiazepines may face life-threatening abrupt discontinuation (
18 ) or be switched to a less desirable alternative (
15,
17 ).
No study has yet estimated the national prevalence of benzodiazepine use in the Medicare population. Furthermore, little is known about the relationship between insurance for prescription drugs and benzodiazepine and substitute medication use and spending. To address these knowledge gaps, we conducted retrospective analyses, focusing on two objectives: to provide national estimates of benzodiazepine utilization and expenditure patterns among Medicare beneficiaries and to examine the impact of prescription drug insurance coverage and other factors associated with utilization of benzodiazepines and potential benzodiazepine substitute classes.
Methods
Data were obtained from the 2002 Medicare Current Beneficiary Survey (MCBS), a nationally representative, longitudinal survey data set of Medicare beneficiaries conducted by the Centers for Medicare and Medicaid Services (CMS) (
19 ). Extensive information on the health care and status of Medicare beneficiaries—including sociodemographic characteristics, medical and prescription drug insurance supplements, annual use, and expenditures for all health services—is collected in the MCBS through person-level surveys and linked to medical claims records. Sampling weights allow generation of population-level estimates. Our study sample consisted of community-dwelling MCBS respondents in the cost and use file in 2002 (weighted N= 32,504,074). The University of Maryland Baltimore Institutional Review Board exempted this study from the board's full review.
Our two primary dependent binary measures designated any annual use of any benzodiazepines or potential substitute drugs. Drug names were identified from the MCBS prescribed medicine event (PME) file, which contains self-reported outpatient prescribed medicine events for each survey respondent. Benzodiazepines used by the 2002 MCBS population included alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, lorazepam, oxazepam, estazolam, flurazepam, quazepam, triazolam, and temazepam. On the basis of descriptions in the PME file, the first seven benzodiazepines were further classified as antianxiety agents, and the last five benzodiazepines were described as sedative-hypnotics. Anxiolytics, including buspirone and meprobamate, were also listed as antianxiety agents and therefore were identified as potential substitutes for antianxiety benzodiazepines. Similarly, nonbarbiturate sedative-hypnotics, including zolpidem, chloral hydrate, zaleplon, and diphenhydramine, were identified as potential substitutes for sedative-hypnotic benzodiazepines. We did not include barbiturate sedative-hypnotics in our study because barbiturates also are excluded from Medicare Part D reimbursement.
The utilization of potential benzodiazepine substitute classes was examined in two subgroup analyses. Group 1 consisted of a population that used either sedative-hypnotic benzodiazepines or nonbarbiturate sedative-hypnotics. Beneficiaries were classified into two mutually exclusive subgroups: those who used sedative-hypnotic benzodiazepines and those who used nonbarbiturate sedative-hypnotics. Beneficiaries who used both drugs were classified into the nonbarbiturate sedative-hypnotics group because this subgroup analysis was intended to mimic the Medicare Part D situation. Because benzodiazepines are not covered under Part D, beneficiaries who used both drugs were assumed to be more likely to not use the uncovered benzodiazepines and continue using the covered nonbarbiturate sedative-hypnotics.
Similarly, group 2 consisted of beneficiaries who used either antianxiety benzodiazepines or anxiolytics. We created two mutually exclusive subgroups, those who used antianxiety benzodiazepines and those who used anxiolytics, with beneficiaries using both drugs classified into the anxiolytics group. A post hoc analysis of beneficiaries who used both benzodiazepines and the potential benzodiazepine substitutes applied three types of categorization—combining beneficiaries who used both benzodiazepines and potential benzodiazepine substitutes with the benzodiazepine group, combining these beneficiaries who used both benzodiazepines and potential benzodiazepine substitutes with the potential benzodiazepine substitutes group, or simply excluding these beneficiaries—all of which showed the same direction and only slight variations in the utilization estimates.
The independent variable of interest was drug coverage, classified as no drug coverage, Medicaid, private health insurance, health maintenance organization (HMO), or other public health insurance plan. If beneficiaries possessed more than one type of drug benefit, they were assigned to a primary source according to the following hierarchy, which is based on relative benefit generosity: private health insurance, HMO, Medicaid, other public insurance, and no drug insurance. Because of sample size limitations in the subgroup analyses evaluating benzodiazepine and potential substitute class utilization, the five drug coverage categories were collapsed into a binary variable to denote any drug coverage, with no drug coverage as the reference.
Covariates included selected chronic conditions treated with benzodiazepines (diagnoses of anxiety, depression, bipolar disorder, psychotic disorders, drug or alcohol abuse or dependence, other psychiatric disorder, or seizure or convulsion). We identified beneficiaries with these conditions on the basis of condition category risk adjusters. Aggregated from
ICD-9-CM codes, condition categories are part of the CMS hierarchical condition category model used to risk-adjust Medicare capitation payments to Medicare Advantage (previously called M+C) plans (
20 ). On the basis of diagnoses recorded on beneficiaries' physician, outpatient, and inpatient Medicare claims, beneficiaries are coded for the most severe diagnosis within each condition category (
20 ). Individuals may have multiple condition categories. We also controlled for demographic variables, including age, sex, and race. Age was classified into four categories: age 64 and under (those who were eligible for Social Security Disability Insurance [SSDI] on the basis of disability), 65–74, 75–84, and 85 years and older. Income was categorized in relation to the federal poverty level (FPL) because Medicaid eligibility is based on this criterion.
Descriptive analyses were conducted to explore patterns of benzodiazepine utilization and expenditures. Multivariate logistic regression analysis was conducted to determine the association between the independent variables and the likelihood of utilization of benzodiazepine and potential substitute drugs. All analyses used the provided weights to allow national prevalence estimates and were conducted using SAS, version 9.1. In addition, we used robust estimators SURVEYFREQ, SURVEYMEANS, and SURVEYLOGISTIC were used to appropriately adjust standard errors and control for the clustering inherent in the complex sampling design of the MCBS.
Results
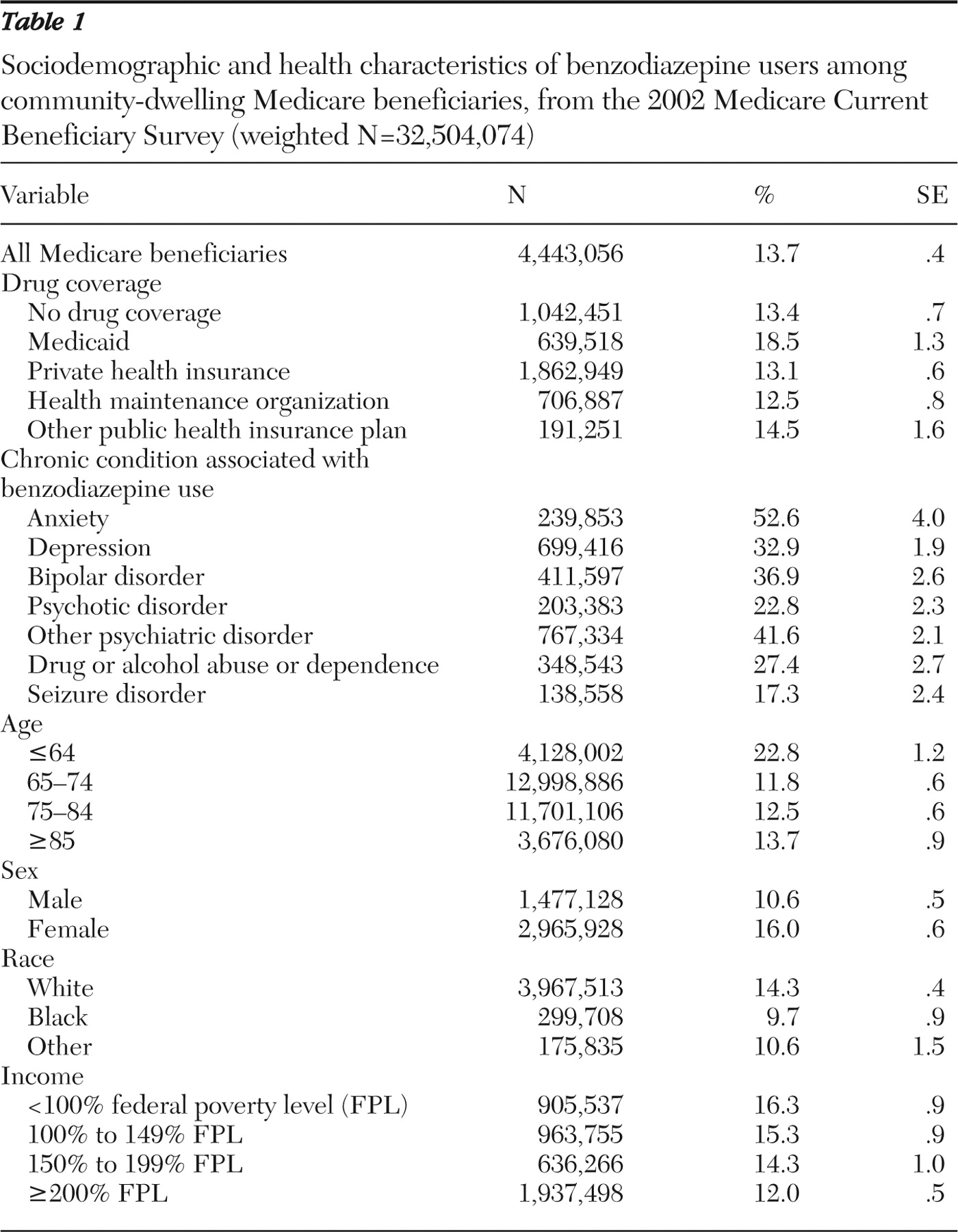
In 2002 over four million, or 13.7%, community-dwelling Medicare beneficiaries received at least one benzodiazepine (
Table 1 ). Among benzodiazepine users, use was highest among beneficiaries with drug coverage from Medicaid (18.5%), whereas 13.4% of beneficiaries without drug coverage and 12.5% of beneficiaries with drug coverage from HMOs used benzodiazepines. More than half (52.6%) of beneficiaries with anxiety and almost one-third (32.9%) of those with depression used at least one benzodiazepine. Prevalence of use was highest among the SSDI-eligible disabled population under 65 years old. In addition, most benzodiazepine users were female and white and had an annual income less than 100% FPL.
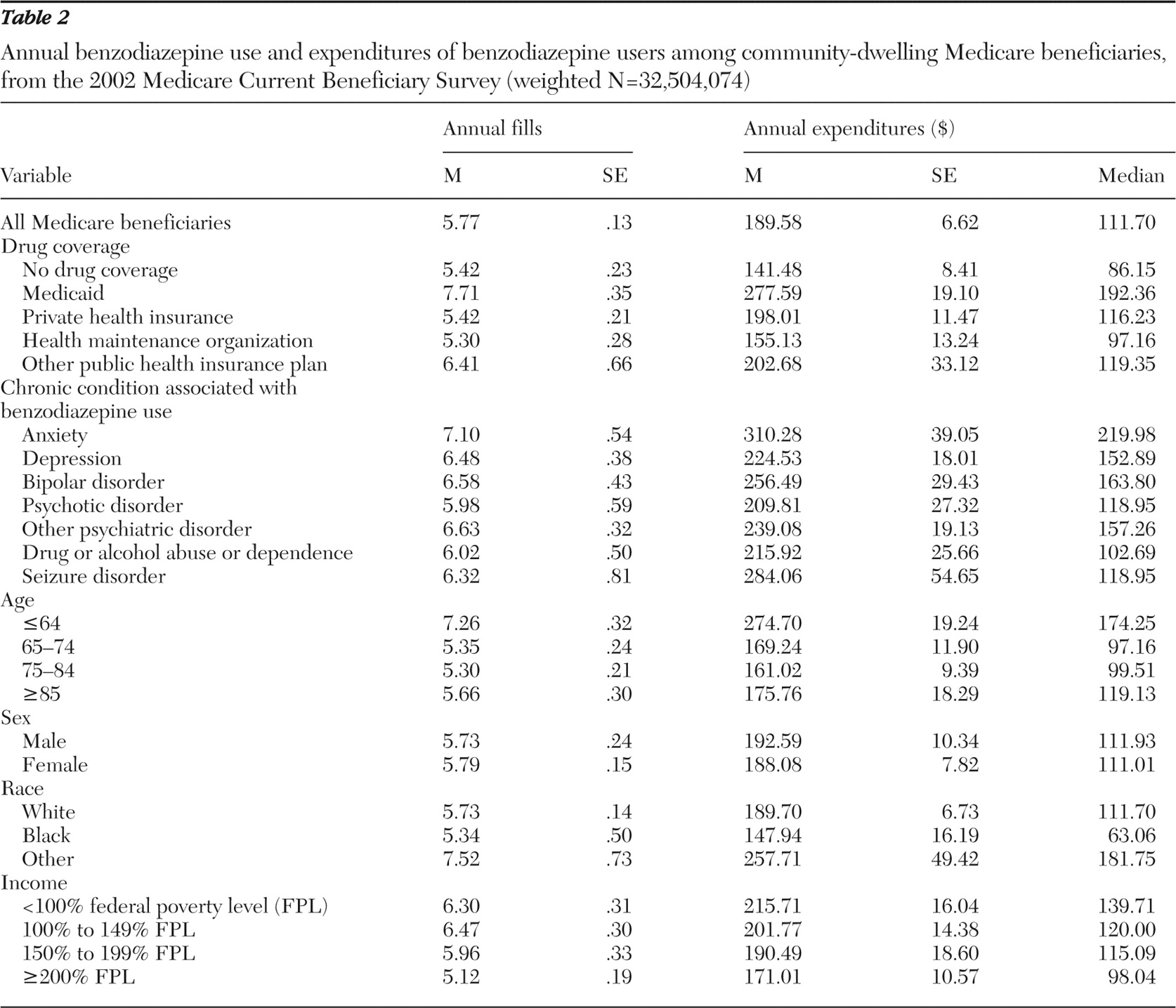
The distribution patterns of annual filled benzodiazepine prescriptions and benzodiazepine users were similar (
Table 2 ). In 2002 benzodiazepine users received an average of 5.77 benzodiazepine prescriptions per person per year. Mean annual filled benzodiazepine prescriptions were highest among those with Medicaid drug coverage (7.71 fills), followed by those with other public plan drug coverage (6.41 fills). Although the utilization of benzodiazepines was quite similar across selected condition categories, beneficiaries with anxiety had the highest number of filled benzodiazepine prescriptions (7.10 fills). In addition, the SSDI-eligible population utilized more prescriptions (7.26 fills) than their older counterparts. Although sex did not make a difference in benzodiazepine utilization, beneficiaries whose race was other than black or white had higher benzodiazepine use (7.52 fills) than white beneficiaries (5.73 fills) or black beneficiaries (5.34 fills). Finally, lower-income beneficiaries used more benzodiazepines (6.30 fills and 6.47 fills for those with income below 100% FPL and income between 100% and 149% FPL, respectively) than beneficiaries with higher income.
Benzodiazepine expenditures generally mirrored trends seen with utilization patterns. In 2002 benzodiazepine users spent $189.58 on benzodiazepines, on average, for the year. The annual mean benzodiazepine expenditure was highest among beneficiaries with Medicaid ($277.59) and lowest among beneficiaries with no drug coverage ($141.48). Beneficiaries with anxiety spent relatively more on benzodiazepines annually ($310.28), compared with those with other chronic conditions associated with benzodiazepine use. Medicare beneficiaries under age 65 spent at least $100 more on benzodiazepines than their older counterparts. Mean expenditures differed only slightly by sex. However, those of other races had higher mean expenditures ($257.81) than did white beneficiaries ($189.70) or black beneficiaries ($147.94). Annual average benzodiazepine expenditures decreased as income increased. Similar to the highly skewed characteristics of most health care cost data, the medians of the annual benzodiazepine expenditures were lower than the means across all categories.
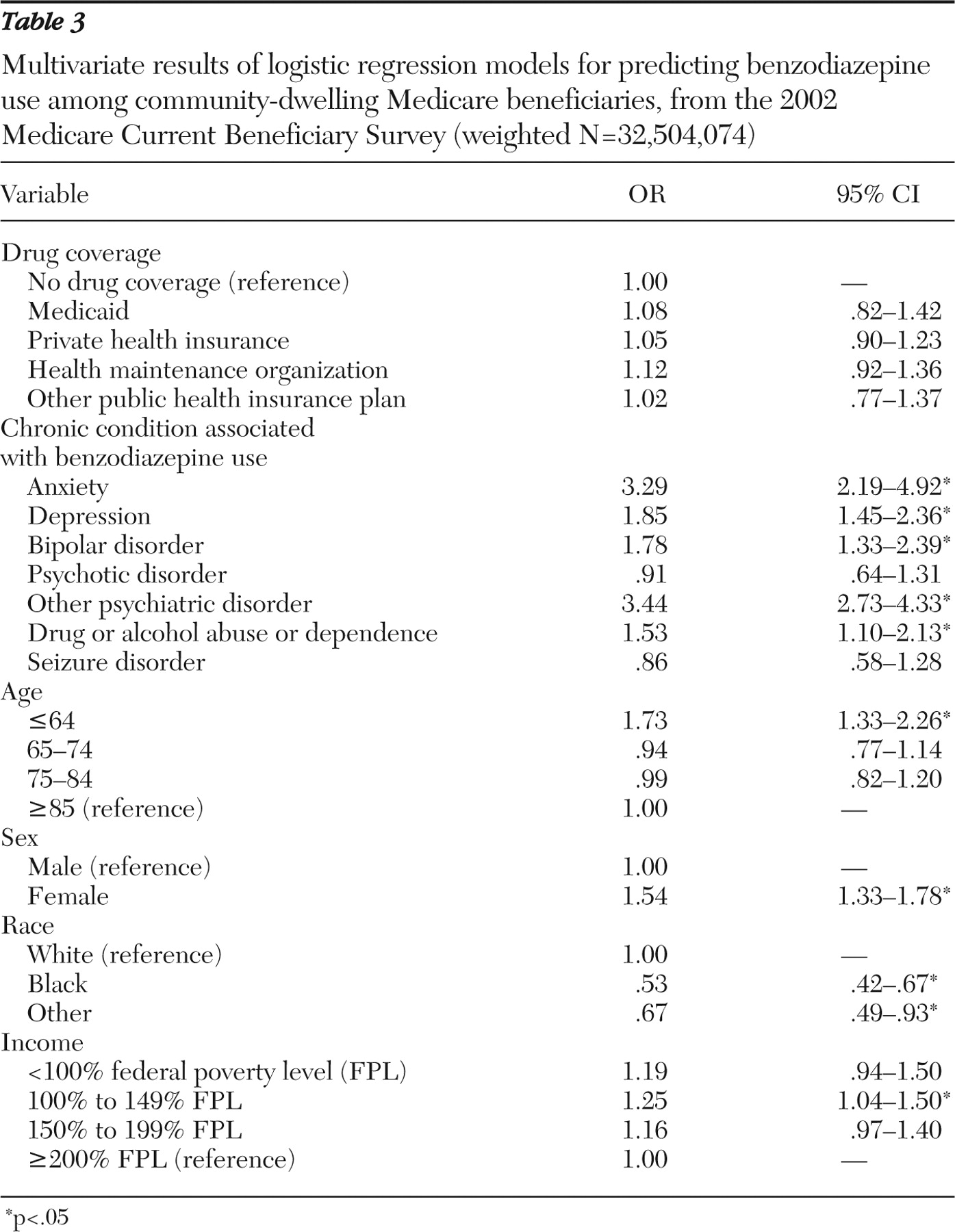
Although increased benzodiazepine use was noted among beneficiaries with drug benefits compared with those without drug benefits, drug coverage, our primary independent variable of interest, was not significantly associated with benzodiazepine use (
Table 3 ). Among chronic mental health conditions, anxiety, depression, bipolar, drug or alcohol abuse or dependence, and other psychiatric disorders significantly increased the odds of benzodiazepine use. Other covariates demonstrating strong and significant associations with benzodiazepine use included being disabled and younger than 65 (odds ratio [OR]=1.73), being female (OR=1.54), having an income between 100% and 149% of FPL (OR= 1.25), and being black (OR=.53) or of another race (OR=.67).
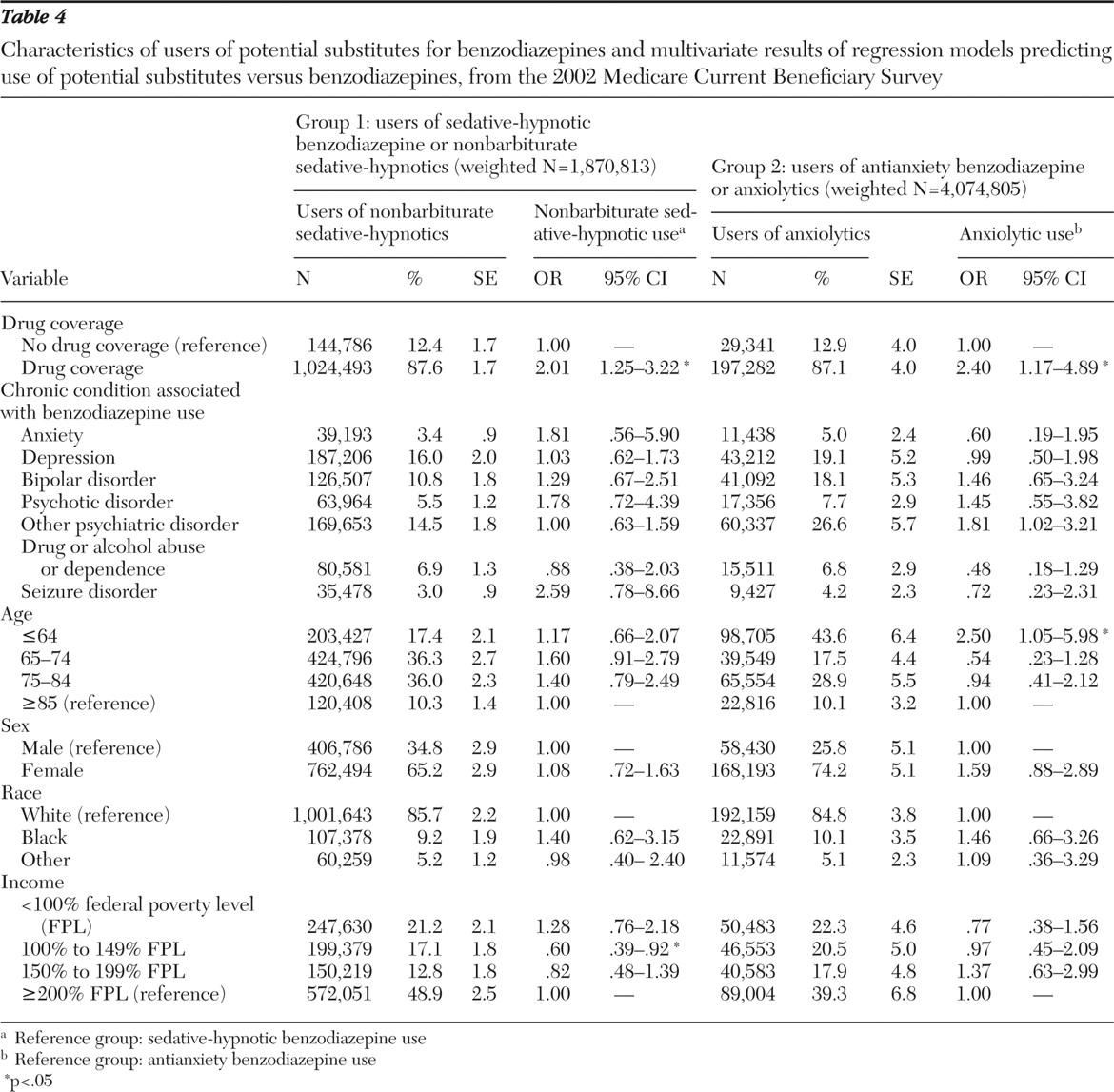
Two subgroup analyses examined use of potential benzodiazepine substitute classes (
Table 4 ). In group 1 (beneficiaries who used either sedative-hypnotic benzodiazepines or nonbarbiturate sedative-hypnotics), descriptive statistics revealed that higher percentages of beneficiaries using nonbarbiturate sedative-hypnotics had drug coverage, depression, other psychiatric disorders, and higher income and were in the older age groups, female, and white. With covariates controlled for, beneficiaries with drug coverage were significantly more likely to use nonbarbiturate sedative-hypnotics (OR=2.01) than sedative-hypnotic benzodiazepines (reference group). In addition, lower income status (100%–149% FPL) significantly decreased the odds of using nonbarbiturate sedative-hypnotics rather than using sedative-hypnotic benzodiazepines (OR=.60) in comparison with the highest income level (≥200% FPL).
Similarly, in group 2 analyses, we compared the effect of each independent variable on the use of other anxiolytics in regard to antianxiety benzodiazepines (reference group). Among beneficiaries using anxiolytics, the proportion of those who had drug coverage, depression, other psychiatric disorders, and higher income status and those who were disabled, female, and white was higher than their benzodiazepine-using counterparts. Furthermore, beneficiaries with drug coverage (OR= 2.40) and who were disabled (OR= 2.50) were significantly more likely to use other anxiolytics than antianxiety benzodiazepines.
Discussion
This study provided information on the national prevalence of benzodiazepine utilization and expenditures in the Medicare population. We also examined potential implications of benzodiazepine and substitute medication use and spending under the current Part D benzodiazepine exclusion policy. Findings showed beneficiaries with disabilities and those with lower income were more likely to use benzodiazepines. Although benzodiazepines are relatively inexpensive, incurring their cost may still present a heavy burden for these vulnerable populations. Consistent with previous research (
21,
22,
23 ), our study also found age, sex, and racial disparities associated with benzodiazepine use. Findings suggest further exploration of age, sex, and racial-ethnic differences among Medicare beneficiaries, especially in the context of medication use under Part D.
The relationship between drug coverage and drug utilization has been widely studied (
24,
25 ), and findings show that reduced or discontinued drug benefits generally are associated with consequent reductions in medication use. Although our study did not report significant associations between benzodiazepine use and specific sources of drug coverage among beneficiaries, persons with drug coverage were more likely to use the potential benzodiazepine substitute classes, as shown in the two subgroup analyses. This finding suggests that under Part D, beneficiaries with less generous drug coverage plans may be affected by the benzodiazepine exclusion policy. Further exploration of beneficiaries' use of potential substitutes for benzodiazepine should focus on payer source to determine how generosity of drug benefit influences drug utilization and spending.
The widespread use of benzodiazepines among beneficiaries with various types of psychiatric conditions, especially anxiety, also was demonstrated. Benzodiazepines are recommended for short-term treatment of anxiety disorders (
12,
26 ). Although gradually switching from benzodiazepines to selective serotonin-reuptake inhibitors (SSRIs) or non–benzodiazepine anxiolytics is recommended for long-term management of anxiety (
18 ), the initial and immediate relief of benzodiazepine therapy is still valuable. Furthermore, although restricting access to benzodiazepines is generally perceived to reduce related risks (
27 ), a recent study suggests that policies resulting in significant reductions in benzodiazepine use in the elderly population failed to decrease the incidence of hip fracture (
28 ).
Although this is the first study to examine benzodiazepine utilization and expenditures associated with source of drug coverage, several study limitations must be addressed. Because we were unable to assess the 2006 data after Part D was implemented, we used the most current MCBS data available (year 2002) at the time of analysis. Therefore, our data might not capture the actual use of benzodiazepines and the full impact of drug coverage because benzodiazepines were still covered by Medicaid and other drug coverage plans back then. Also, prescribing trends likely have changed since then.
The MCBS has notable limitations. For one, the survey does not allow reliable daily dose estimates nor estimates of duration of use. Several variables in the MCBS were not reliable enough to provide information on days' supply and date of dispensing; thus we could not assess issues such as appropriateness of benzodiazepine use in this population. We also could not assess the barrier of drug copayments to the beneficiaries, because the MCBS data set lacks reliable data on drug copayments. Furthermore, we recognize that anxiety often occurs with other psychiatric disorders, including depression and other mood disorders, and as such, anxiety may be underdiagnosed and underreported in MCBS data. The algorithms used in assigning individuals to specific condition categories were based on at least one diagnosis regardless of provider service; thus assignment to condition categories may result in a more heterogeneously defined clinical population than one identified on the basis of inpatient versus outpatient claims or two or more diagnostic claims. Nevertheless, the condition categories are highly validated risk adjusters (
20 ).
In our subgroup analyses of the utilization of potential benzodiazepine substitutes, although we would have liked to include selected antidepressants, such as the SSRIs, as one of the substitutes for the population using antianxiety agents, the use of SSRIs is driven by diagnoses of depression, rather than anxiety, and therefore would have dominated the study results. Furthermore, the indication of anxiety disorders, including generalized anxiety disorder, panic disorders, obsessive-compulsive disorder, and posttraumatic stress disorder, were not approved for most SSRIs, although off-label use for these conditions is common. Considering that Food and Drug Administration approval of SSRIs for anxiety was given between 2001 and 2003, our data would have reflected off-label use.
Despite the discussed limitations, this study provides a benchmark for benzodiazepine utilization and expenditures before implementation of Part D. Future studies are needed to examine benzodiazepine use after Part D implementation to further explore how drug coverage in general, and source in particular, influences access to benzodiazepines. Additional work is needed to analyze the impact of drug coverage changes on discontinuation of benzodiazepines as well as of switching to more potent or more expensive therapeutic substitutes, including sedative-hypnotics, antidepressants, and select antipsychotics with sedative properties.
Conclusions
In 2002, 13.7% of Medicare beneficiaries received at least one benzodiazepine, with a higher level observed among those with anxiety and other chronic mental health conditions. Disabled persons and beneficiaries with lower incomes used significantly more benzodiazepines than other beneficiaries. Sex and racial disparities also were associated with benzodiazepine use. Compared with having no drug coverage, having drug coverage significantly increased the odds of using potential substitute medications over benzodiazepines.
Acknowledgments and disclosures
Dr. Zuckerman was supported by Research Scientist Development Award K01-AG-022011 from the National Institute on Aging. The authors gratefully acknowlege Thomas Shaffer, M.H.S., for data assistance.
The authors report no competing interests.