Recent policy concern has focused on the overuse of prescription drugs (
1–
10). Overprescribing is a public health concern because it may result in side effects and adverse health outcomes, as well as waste the limited financial resources of individuals and the health care system (
1,
8,
10,
11). Improving the efficiency of our health care system is an imperative now that an expected additional 57 million Americans will become insured under the 2010 Patient Protection and Affordable Care Act (PPACA) (
12). Recent medication overuse research has focused on estimates of overuse of nonsteroidal anti-inflammatory agents, cox-2 inhibitors, and proton pump inhibitors among adults and overuse of antipsychotics among the elderly population (
2–
6,
9).
Antidepressant prescribing in the United States has increased over time (
13–
15). It has been suggested that the rise in antidepressant usage among adults coincides with the introduction of selective serotonin reuptake inhibitors (SSRIs), perceived as advantageous over the older tricyclic antidepressants (TCAs) because of enhanced safety and more tolerable side effect profiles (
8,
15–
21). The prevalence of antidepressant prescribing, coupled with the sharp rise in use in recent years, has led some to question whether antidepressants are overused (
2,
5,
8,
22).
Yet, interpreting the public health impact of increasing antidepressant usage among adults is complicated. The problems of underdiagnosis and undertreatment of depression are a major public health concern, and measures have been taken to increase public awareness, improve systematic screening, and implement evidence-based treatment guidelines (
23–
26). As a consequence, increased utilization may be viewed as evidence suggesting that depression and other mental disorders are becoming better identified and treated through advances in the availability of more tolerated pharmacological treatments (
8,
16,
27).
There are limited empirical data to support the claim that antidepressants are overused in the U.S. adult population. Epidemiologic surveys have been used to bound estimates of overuse of mental health treatments. Results suggest that approximately half of respondents who obtained treatment for mental or substance use disorders in a study year did not meet criteria for any disorders assessed (
23,
28,
29). Recent analyses suggest that these overuse estimates are likely biased upward (
11). Furthermore, none of this prior work provides overuse estimates for specific treatment modalities, such as prescription antidepressants (
26,
30).
To our knowledge, there is one published study that provides a direct estimate of antidepressant overuse among American adults (
2). Using the National Drug Therapeutic Index, which surveys a nationally representative sample of office-based physicians, the authors examined overuse of two older-generation TCAs (amitriptyline and nortriptyline)—medications with greater risk of complications and lower tolerability than many of the newer antidepressants (
2). The authors defined overuse as off-label antidepressant prescribing for diagnoses for which the drug had limited or no scientifically supported applicability according to the
Physicians' Desk Reference, the
United States Pharmacopeia-National Formulary, and the Micromedex DrugDx data system. They determined that these medications were used to treat indications not approved by the U.S. Food and Drug Administration (FDA) approximately 60%–80% of the time and in clinical contexts with little or no scientific support approximately 60% of the time. However, the data for that study were from 2001, and whether or to what degree the results are applicable to current practice is unclear, given the fact that newer-generation antidepressants, such as the SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs), have become prominent in therapy (
13,
14).
The objective of this study was to provide a more comprehensive and recent estimate of antidepressant overuse in a nationally representative survey of adults. Prescriptions for all older- and newer-generation antidepressants among adult respondents to the Medical Expenditure Panel Survey (MEPS) were identified by clinical indication and determined to be on label (FDA approved), off label (lacking FDA approval) with scientific evidence of therapeutic efficacy, or off-label without supporting evidence—which we considered to be overuse. The approach was consistent with that of Radley and colleagues (
2) and with additional studies of overuse of other medications (
3,
6). We report the magnitude of antidepressant overuse overall and by class of specific medications used. We classified documented clinical indications for overuse by using sample stratification. We used multivariate regression to examine socioeconomic and subclinical predictors of overuse consistent with previous reports of underuse and overuse (
11,
14,
20,
23–
27,
30–
32).
Methods
The Harvard Medical School Institutional Review Board approved the study. Stata version 9 software was used for statistical analyses (
33).
Data source
Self-reported data on antidepressant usage and its indication were drawn from the household component (HC) and prescription drug component (PDC) of the 2005 MEPS (
34,
35). Conducted since 1996, the MEPS is a nationally representative annual survey of approximately 15,000 households that is fielded by the Agency for Healthcare Research and Quality (AHRQ); it has been described in previous studies of population-based estimates of medication use, including use of antidepressants (
13). In the MEPS-HC, persons from selected households are interviewed face to face three times in each survey calendar year to obtain health care utilization information.
Self-reported diagnoses linked to prescriptions may be subject to reporting biases. To minimize this, the MEPS subjects the self-reported diagnoses and drug usage reported in the MEPS-PDC to validation procedures (
34–
36). In each interview, respondents are asked to record in a calendar or diary any medical events that occur subsequent to the previous interview. After written permission is obtained from respondents, MEPS survey staff contact each listed medical provider to supplement and validate diagnostic and other clinical information about reported medical visits. The MEPS-PDC collects information on all prescribed medicines associated with each health care visit, including the names of medicines purchased or otherwise obtained, the first and last dates taken, the number of times the prescription was filled at the pharmacy, and the conditions associated with each medicine. These responses are validated through the collection of more detailed information on prescriptions filled at local pharmacies, including type, dose, and payment.
Additional sources of self-report bias may occur in the MEPS-HC for the following reasons: respondents may be reluctant to report mental health problems, respondents may have imprecise knowledge of their diagnoses (for example, respondent self-report of depression may not be consistent with a depressive disorder diagnosis by a health care provider), and respondents are asked to report on diagnoses of family members for whom they may have imprecise information (
35,
36). We took several steps to minimize these concerns in our analyses. First, prior validation research conducted on the MEPS demonstrated that when compared with the MEPS Medical Provider Component, considered the gold standard for diagnostic accuracy, MEPS-HC was found to be highly accurate for mental health and substance abuse conditions, broadly speaking (87.7%), and when establishing broad diagnostic categories (such as depressive or anxiety disorders [88.2%–93.4%] rather than specific depressive or anxiety disorder diagnoses) (
36). Therefore, we used the broader diagnostic categories of mental health conditions suggested in prior research in analyses. Second, we excluded from analyses surveys completed by proxy or by family member.
Sample selection
We identified all adult nonproxy respondents (aged 18 and older) in MEPS-HC who had an antidepressant prescription in 2005. As with most surveys, the MEPS-HC participants represent only a fraction of the civilian noninstitutionalized U.S. population the survey is intended to reflect. In order to calculate estimates representing the national population, AHRQ suggests that responses from surveyed individuals in the MEPS-HC be weighted by the proportion of the population they represent, with results adjusted to account for nonresponse (
34–
37). Consequently, each MEPS-HC file contains weight variables that may be applied to the data to generate national estimates of the civilian noninstitutionalized population. A detailed description of the weighting process and how weights should be used in estimation may be found in the weighting and estimation section of the MEPS-HC 2005 Full Year Consolidated Data File (
37). In this study, MEPS-HC survey weights were used in all analyses, and results were corrected for the complex sampling scheme consistent with these recommendations (
37). The final weighted sample of all antidepressant users corresponds to 23,026,608 adults.
We pursued two sensitivity analyses to assess the importance of sample selection and year selection to our findings. First, we assessed patterns of antidepressant use and overuse only among adults who self-reported being “new users of this medication” in 2005. The weighted sample of new users corresponded to 7,931,219 adults. Second, we assessed patterns of adult antidepressant use and overuse among all users and new antidepressant users in the 2002 MEPS. The weighted 2002 sample corresponded to 20,345,404 overall users and 8,909,670 new users of antidepressants.
Clinical indications for antidepressant use
We linked antidepressant prescriptions to clinical indication at the individual patient level. On the basis of the broad diagnostic categories suggested by Machlin and colleagues (
36), we used primary, secondary, and tertiary four-digit
ICD-9-CM codes in the MEPS to identify antidepressant-indication matches (
38). We used the Multum database to identify specific clinical indications based on these codes (
39). Multum has been used for this purpose previously and has been shown to have a high degree of matching accuracy between
ICD-9-CM codes and diagnoses (
40). Diagnoses assigned to each antidepressant prescription were reviewed independently by two authors (RC and ABB) and by an independent physician board-certified in internal medicine. The intraclass correlation coefficient measuring the consistency between the two raters who are authors was high (.89). An independent psychiatrist with expertise in the treatment of mood disorders reviewed discrepancies in the authors' attribution of prescription to indication. Discrepancies were resolved by consensus.
Strength of classification of antidepressant medications
We classified prescription-indication pairs into the following three categories: FDA-approved use, off-label use with strong scientific support based on effectiveness studies, or off-label use with limited or no scientific support—which we defined as overuse. Indications were considered to be FDA approved if they could be matched to therapeutic indications reported in the drug's package insert (compiled in the
Physicians' Desk Reference) or were listed as approved indications in the
United States Pharmacopeia-National Formulary (
41–
43). Use with no recorded diagnosis or refill administrative codes was categorized as overuse. For individuals with two or more diagnoses recorded (10%), use was considered FDA approved if at least one of the diagnoses recorded indicated an approved use.
The degree of supporting evidence for each off-label indication was assessed using the Micromedex DrugDx System, a nationally recognized pharmaceutical compendium that describes the efficacy and scientific documentation for on- and off-label uses of prescription drugs (
44). It is used to approve payment for off-label use by Medicaid and Medicare and has been used by previously published studies estimating medication overuse (
2,
43).
On the basis of published studies, we applied medication-specific indications to the therapeutic class given the evidence supporting antidepressant treatment for diagnoses for which an agent in the same or related class has received FDA approval (for example, SSRIs in the treatment of anxiety disorders) (
18,
19,
36,
45). On the basis of this criterion, usage of the TCAs, SSRIs, and the SNRIs was considered off label with strong scientific evidence for the treatment of a variety of pain-related conditions, including headache, neuropathy, and neuralgia, and usage of the SSRIs and SNRIs was considered off label with strong scientific evidence as aids in the treatment of a variety of anxiety disorders. [A table that lists antidepressants, their uses, and their degree of scientific support is available as an online supplement to this article at
ps.psychiatryonline.org.]
Data analysis
We report antidepressant use and overuse overall and by class of specific medications used. To provide more detail regarding clinical circumstances associated with antidepressant overuse, we used descriptive statistics and stratification to classify the most common primary ICD-9-CM diagnoses associated with overuse.
Previous studies have suggested that undiagnosed mental illness (particularly among men and certain ethnic subgroups), lack of insurance, and low income may be predictors of the underuse of antidepressants and other mental health treatment modalities (
11,
14,
20,
23–
27,
30–
32). We fit a multivariate logistic regression model to examine predictors of overuse, including age, gender, self-reported race-ethnicity, education level, income group, and insurance status (
11,
14,
20,
23–
27,
31,
32). We included a combined measure of fair to poor general health distinct from diagnosis and used self-reported responses on a 5-point scale (rated from 1, excellent, to 5, poor) from the validated Physical Health Index (PHI) derived from the 12-Item Short Form Health Survey (SF-12) (
46). Mental health need distinct from a diagnosis was measured with the Kessler 6-Item Psychological Distress Scale (K6), a measure of generalized distress that has shown good agreement with the Composite International Diagnostic Interview in general adult population surveys (
47). Odds ratios (ORs) predicting the overuse of antidepressants in this population were constructed with the use of robust standard errors adjusted for clustering at the individual level (
48).
Results
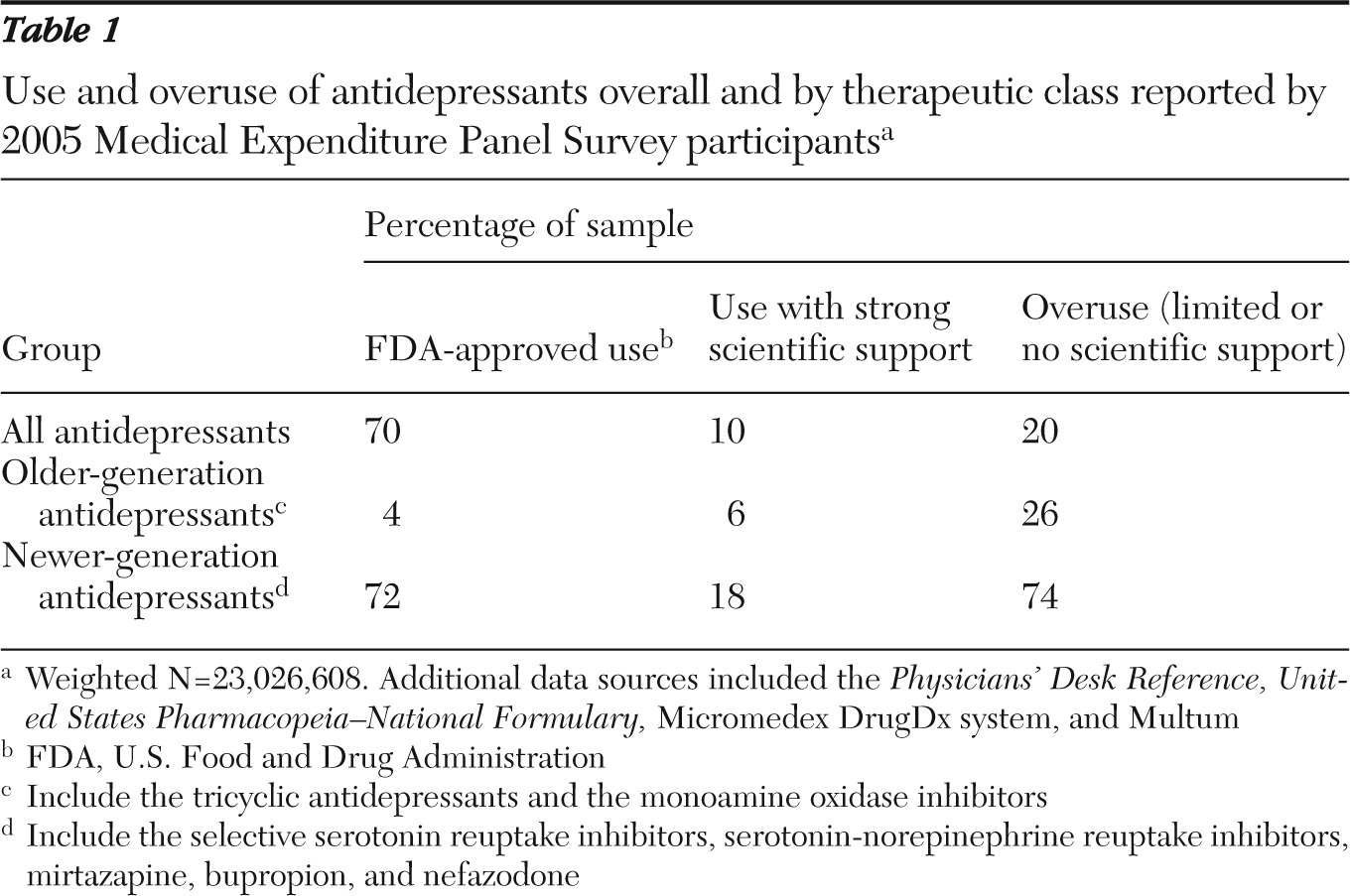
We found either no diagnostic rationale or an insufficient one in one-fifth of antidepressant use over all antidepressants (20%) (
Table 1). Our sensitivity analyses (the 2005 estimate for new antidepressant users only and 2002 estimates for all users and new users) yielded similar results (22%–23%). Newer-generation antidepressants accounted for most of the use and overuse (90% of use and 74% of overuse). SSRIs were the most prevalently prescribed and accounted for the majority of overuse among newer antidepressants (64% of overuse of newer-generation antipsychotics). TCAs were the most prevalently prescribed older-generation antidepressants and accounted for 92% of older generation overuse.
Persons with diagnoses possibly associated with an undiagnosed or subthreshold mental illness (including general symptoms and diagnoses related to other mental disorders) accounted for common rationales for our definition of antidepressant overuse (general or ill-defined symptoms, 15%; other mental health conditions, 13%; possible depression treatment or prophylaxis, 3%) (
Table 2). Other common rationales were missing diagnoses (18%) and administrative rationales without diagnoses (13%). The results of multivariate logistic regression estimation suggest that older age (OR=.95, p=.03) and poor mental health (OR=.80, p=.02) were negatively associated with antidepressant overuse (
Table 3).
Discussion
In a nationally representative sample of U.S. adults, we estimated that antidepressant overuse occurred with 20% of all users in 2005 and was concentrated in the use of newer antidepressants. These estimates were stable to alternative sample and year selection criteria. They may also be inflated, given that general symptoms, other mental illness diagnoses, and administrative rationales were commonly recorded rationales in our estimate of overuse. We also found that overuse was correlated with clinical characteristics suggestive of alternative measures of need. This finding is consistent with the ample evidence in usual care settings of underdetection and underrecording of psychiatric illness and treatment and recent evidence that most mental health and substance abuse treatment services are provided to people with indicators of need, regardless of formally recorded diagnosis (
11,
20,
23–
27). Being 65 and older was negatively associated with antidepressant overuse in our study. Other recent research using the MEPS has found that older age is negatively associated with antidepressant use (
14).
Using a similar definition of overuse, our antidepressant overuse estimates were similar in magnitude to overuse documented for other commonly used medications among adult Americans, such as the nonsteroidal anti-inflammatory agents and proton pump inhibitors, and less than that documented for antipsychotic usage by the elderly population (
3–
6). We found that newer-generation antidepressants accounted for most of the use, and therefore—not surprisingly—they accounted for most of the overuse. Older-generation antidepressants represented a smaller but not insignificant amount of overuse—26%. Our estimate of older-generation antidepressant overuse was smaller than that found by Radley and colleagues (
2). Part of this discrepancy may be attributable to methodological differences related to sampling frame.
Our approach to estimating antidepressant overuse among adults has several strengths. First, we used validated, internally consistent, self-reported, and nationally representative survey data that matched antidepressant prescriptions to primary, secondary, and tertiary diagnoses at the individual patient level in more recent data. We limited our sample to nonproxy survey respondents for whom self-reported antidepressant usage and diagnostic rationale appeared to be reliably assessed. In determining overuse among newer and older antidepressant classes, we included off-label uses with strong scientific evidence supporting use consistent with clinical practice, Centers for Medicare and Medicaid Services' coverage and payment policies, and previous estimates of overuse for other medication classes.
However, there are some limitations to consider in generalizing the results of this study. First, the MEPS-HC and MEPS-PDC have diagnostic sensitivity limitations (
34–
36). As described in the Methods section, we took several analytic steps to minimize this limitation, including aggregating data over major and minor depression diagnoses and collapsing specific medications into therapeutic classes. Second, data limitations did not allow us to examine other critical factors that might predict overuse of antidepressants, such as cost-sharing arrangements associated with prescription drug use and promotional activities that may influence prescribing practices (
11,
31,
32). Expanding data collection efforts in publicly available data sources to include a richer set of demographic, financing, and organizational correlates of mental health treatment is an important priority. Third, patterns of prescribing antidepressants among the general adult outpatient population may have changed since 2005. However, there have been no major changes in antidepressant options or expert guideline recommendations since 2005, and the results of our sensitivity analyses suggest limited change in overuse between 2002 and 2005.
Together, these results suggest that large changes in antidepressant prescribing between 2005 and 2010 are unlikely. However, expansion of the U.S. population's access to health care services through the 2010 PPACA, including diagnosis and treatment of mental disorders treated with pharmacotherapy, could affect the applicability of these estimates to predicting future rates of antidepressant use and overuse (
12). Therefore, it is important for future study to examine the effect of PPACA on the level and changes in rates of appropriate and inappropriate medication prescribing. Finally, it is possible that a proportion of participants who had an appropriate diagnostic rationale for antidepressant use were in fact misdiagnosed and prescribed an antidepressant in a clinical circumstance in which it would not be efficacious (
49). However, given the well-documented evidence that mental disorders are underdiagnosed in primary care, we believe that in these data, underdetection is likely to be a more common problem than overdetection (
11,
23,
24).
Our results have important implications for quality of care. First, overuse appears to be concentrated in prescriptions for newer medications, primarily SSRIs, with large therapeutic ranges and better side-effect and safety profiles than those of older-generation antidepressants such as TCAs and monoamine oxidase inhibitors. Possibly this is because physicians who are faced with suffering patients with unclear diagnostic syndromes may feel that the potential risk-benefit ratio of these newer medications favors their use in less clear diagnostic circumstances. These results suggest that on the basis of safety concerns, efforts to improve quality of care could focus on further reducing the overuse of older-generation antidepressants. Reducing overuse among all antidepressant classes is an important clinical and policy goal to reduce wasted resources and to reduce unnecessary side effects among patients. Furthermore, future quality improvement efforts should consider that antidepressant overuse among adults was associated with clinical and administrative rationales related to mental health need. These results suggest that administrators aiming to reduce apparent overuse for cost and quality purposes should be cognizant that there are multiple rationales for diagnostic uncertainty in commonly available data of pharmaceutical usage. Third, although overuse is an important quality concern, the health consequences and societal costs of underdiagnosis and treatment are likely to be greater (
1,
7,
11,
20,
23–
25). An ideal medical system's quality improvement efforts would reduce overused care even as it increased underused care.
Conclusions
Our analyses provide empirical evidence that antidepressants appear to be overused less frequently than previously reported, and given the limitations of the data, it is likely that our estimates represent an upper bound of overuse. Nationally representative data collection efforts concerning prescription drug use should aim to include enhanced measures of need to better estimate overuse and to accurately reflect the current state of financing and organization of mental health treatment.
Acknowledgments and disclosures
The study was funded by the Ruth L. Kirschstein Predoctoral Fellowship (T32) from the National Institute of Mental Health. The funding sources had no role in the study's design, conduct, and reporting. The authors thank Andrew Nierenberg, M.D., Ph.D., Nancy Keating, M.D., M.S., Richard G. Frank, Ph.D., and Joseph P. Newhouse, Ph.D., for comments and suggestions.
Dr. Cutler reports being on the speaker's bureaus of Coresource, Chaindrugstore.net, Health Care Financial Management Association, IMS, MedImpact, Medtronic, National Association of Chain Drug Stores, Senate President's Forum, Society of Actuaries, Thomson-Reuters, and U.S. Oncology. He has also been on the advisory board of Genentech. The other authors report no competing interests.