In June 2003, the U.S. Food and Drug Administration (FDA) issued a statement recommending against the use of paroxetine to treat depression of children and adolescents (
1). The statement followed a report suggesting a possible increased risk of suicidal behaviors and suicide attempts (suicidality) associated with this antidepressant among pediatric patients with depression (
1). In October 2003, after preliminary review of results from randomized trials of eight antidepressive agents (citalopram, fluoxetine, fluvoxamine, mirtazapine, nefazodone, paroxetine, sertraline, and venlafaxine), FDA issued a public health advisory about a potential excess risk of suicidality associated with the use of those antidepressants for pediatric patients with major depressive disorder (
2). In the advisory, FDA emphasized that these medications should be used with caution and recommended closer supervision by the treating physicians.
Several studies have used administrative claims databases and automated pharmacy dispensing records to assess the impact of these actions on the patterns of treatment for depression in the U.S. pediatric population (
5–
11). In general, antidepressant utilization among children and adolescents has decreased (
6–
8,
10,
11). Among the studies that examined utilization patterns by antidepressant classes, some found that the use of selective serotonin reuptake inhibitors (SSRIs) declined the most (
5–
7,
10). A recent FDA study using retail dispensing data observed a decrease in antidepressant use in all age groups under 25 years old (ages one to five, six to 12, 13 to 17, and 18 to 24 years) following the 2003 paroxetine advisory (
12). Paroxetine use decreased across all age groups, including among persons 25 years and older, but use of SSRIs other than paroxetine increased, most notably among those ages six to 17 years. Although some studies suggested that changes in treatment patterns were less pronounced for adults (
11), others found that the FDA advisory might also have had an impact on antidepressant utilization in the adult population (
8,
13).
Although studies analyzing dispensing data suggest a decline in overall antidepressant utilization among children and adolescents after the FDA warnings, knowledge about changes, if any, in physicians' prescribing behaviors is still limited. Pharmacy dispensing records might not capture prescribing behaviors because multiple factors determine whether a prescription is filled (
14–
16). In this study, we examined the trends in prescribing antidepressants in ambulatory settings for pediatric patients before and after the FDA advisory, using nationally representative data from surveys of office-based and outpatient-based visits for medical care in the United States. For comparison, we also assessed these trends in the adult population.
Methods
Data source
We used the annual data between 1998 and 2007 from the National Ambulatory Medical Care Survey (NAMCS) and the National Hospital Ambulatory Medical Care Survey (NHAMCS) outpatient department component (
17). These two surveys are conducted annually by the Centers for Disease Control and Prevention to assess ambulatory health care utilization in the United States. NAMCS surveys office-based physician practices, whereas NHAMCS captures services offered at outpatient departments and emergency rooms at hospitals.
NAMCS contains records of physician-patient encounters or visits to the offices of nonfederally employed physicians. The physician or staff fills out information about the patient and provider, reasons for the visit, physician's diagnoses, medications prescribed, and procedures performed. Similar information is collected by NHAMCS for visits to the emergency and outpatient departments of noninstitutional general and short-stay hospitals. Both surveys contain up to three diagnoses for each visit. NAMCS further collects information on provider specialty. The surveys utilize a multistage probability sampling design. Each visit is given a weight that is calculated based on the probability of selection, response rates, and the physician specialty distribution. Upon weighting, these two surveys yield national estimates of health care utilization in ambulatory settings in the United States.
Study population
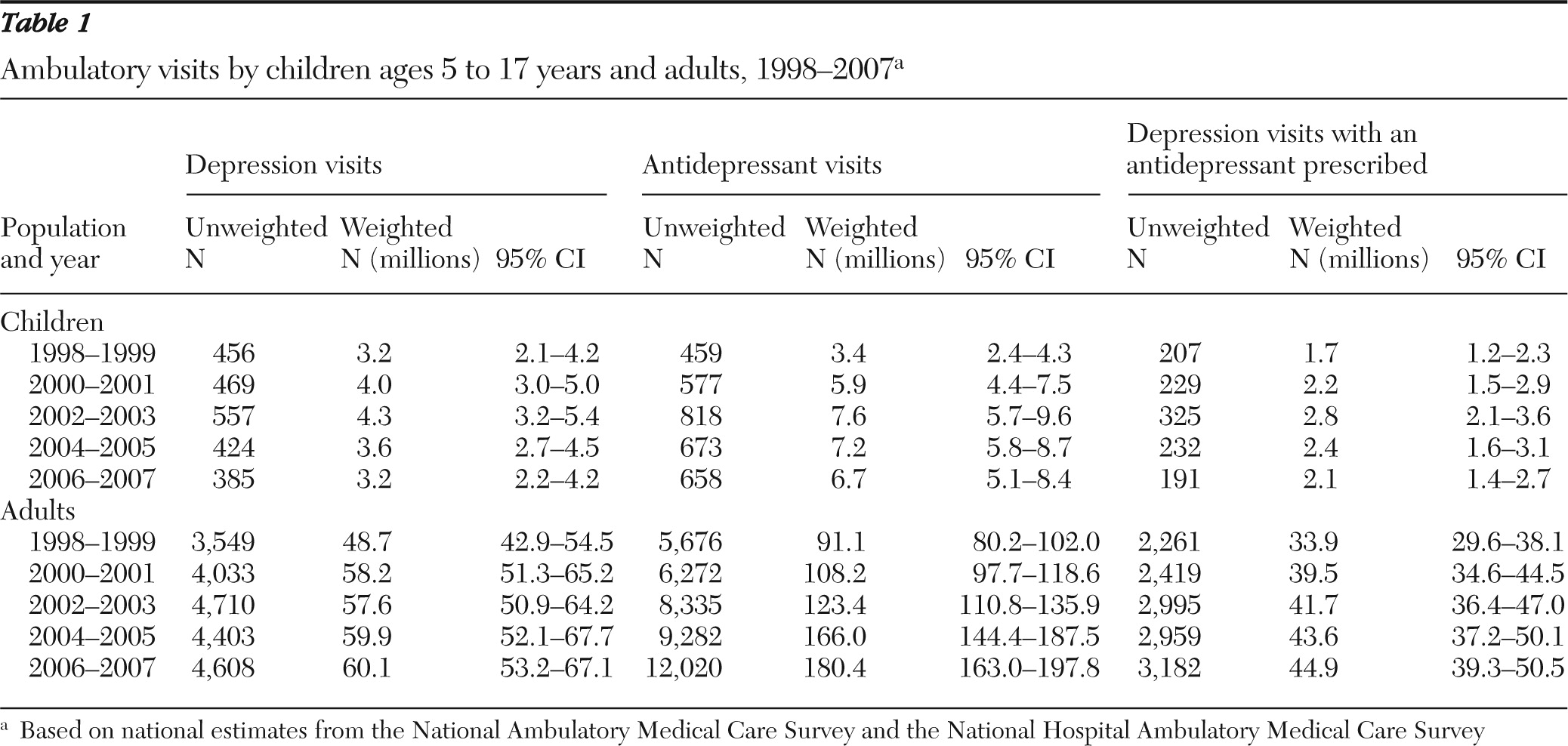
We identified visits made by children aged five to 17 years and by adults aged 18 years and older. We defined depression visits as visits in which any of the diagnosis fields was coded according to ICD-9-CM for major depressive disorder (296.2x or 296.3x), dysthymia (300.4x), or depression not otherwise specified (311). Antidepressant visits were defined as visits in which any antidepressant was prescribed—including SSRIs, tricyclic and related antidepressants, serotonin-norepinephrine reuptake inhibitors, and other antidepressants (trazodone, bupropion [excluding products commonly prescribed for smoking cessation], nefazodone, and mirtazapine). We further identified depression visits with an antidepressant prescribed.
Statistical analysis
We combined data from NAMCS and from NHAMCS's outpatient component to represent all visits in ambulatory settings. Following the analytical guidelines by the National Center for Health Statistics at the Centers for Disease Control and Prevention (
17), we combined data from two consecutive years (1998–1999, 2000–2001, 2002–2003, 2004–2005, and 2006–2007). We considered 2004–2005 and 2006–2007 as the postadvisory period and all other two-year intervals as the preadvisory period.
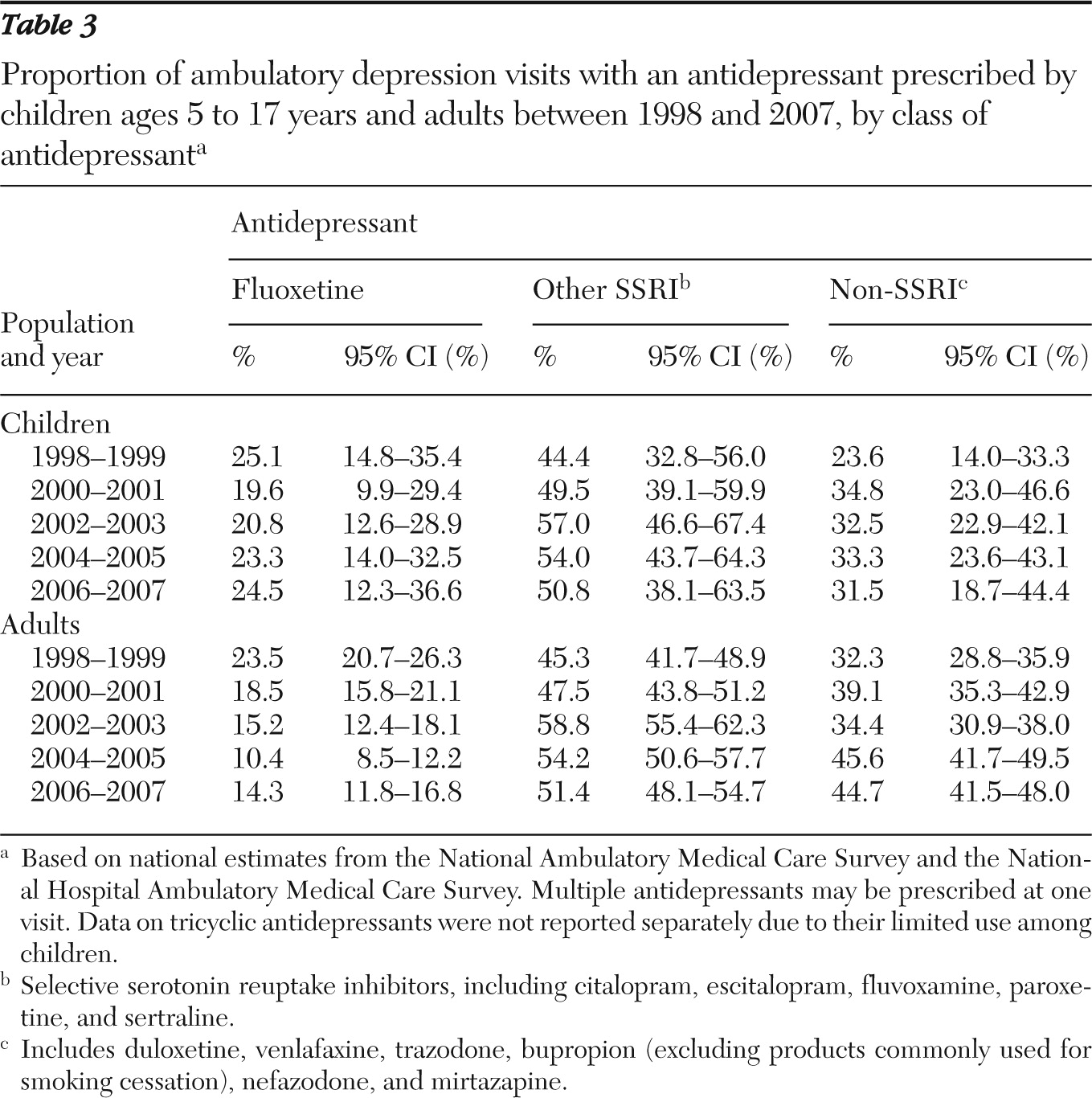
We reported national estimates for the total number of depression visits, the total number of antidepressant visits, and the total number of depression visits with an antidepressant prescribed separately for children ages five to 17 years and adults 18 years and older. Because the underlying ambulatory utilization may have changed over time, we also calculated the proportion of depression visits and of antidepressant visits among all visits made by each group. Similarly, we reported the proportion of depression visits with an antidepressant prescribed among all depression visits as well as among visits that did or did not involve a diagnosis of major depressive disorder. To detect any shift in the use of classes of antidepressants, we examined patterns of use separately for fluoxetine, SSRIs other than fluoxetine, and non-SSRI antidepressants. We separated fluoxetine from the other SSRIs because it is the only antidepressant approved by FDA for pediatric depression (
18). We did not examine use of tricyclic antidepressants separately because their low utilization among children precluded reliable estimation of utilization patterns.
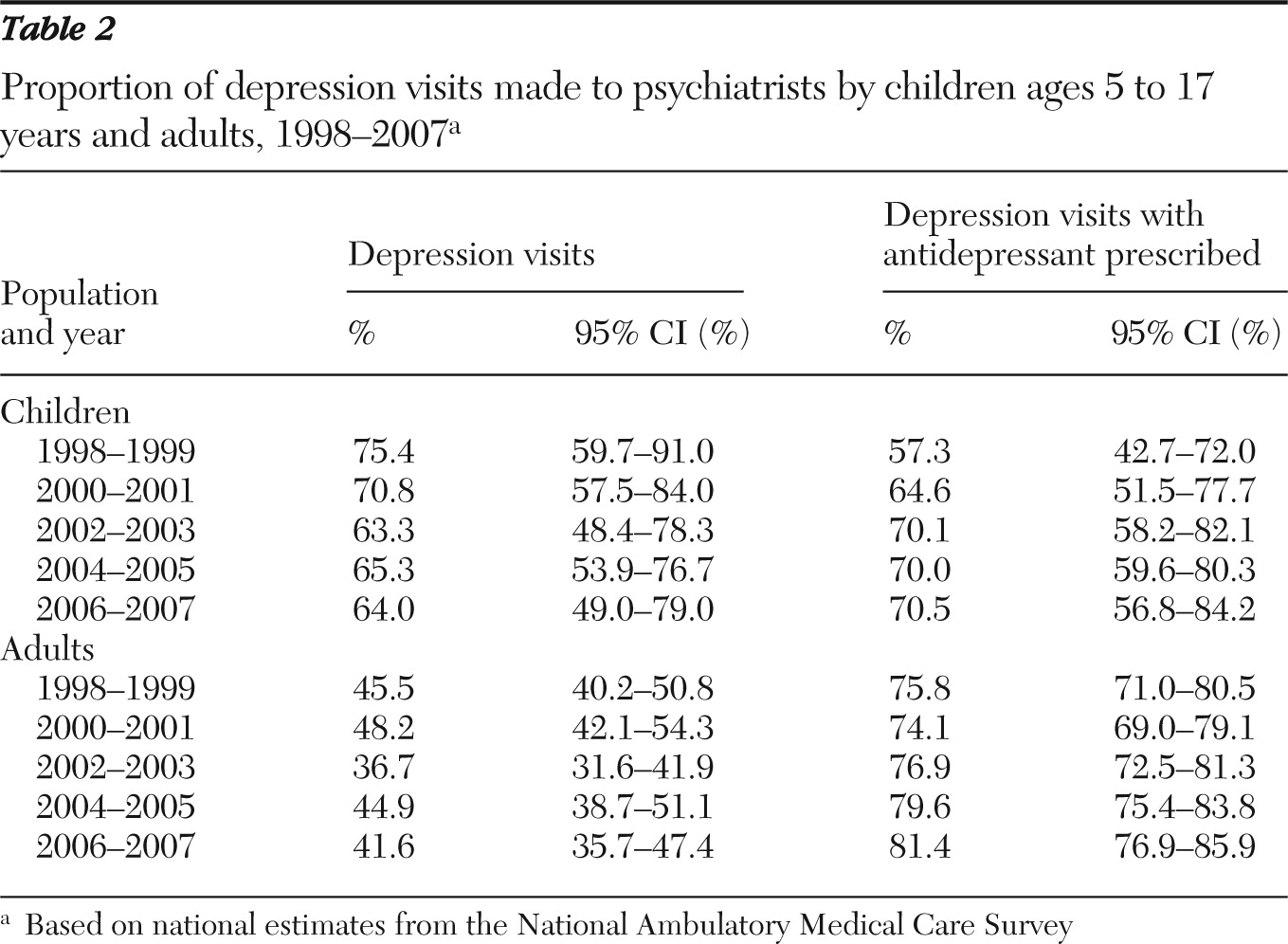
We also performed an analysis by provider specialty using data from NAMCS. We estimated the proportion of depression visits and depression visits with an antidepressant prescribed made to psychiatrists. Stable estimates among nonpsychiatrists could not be obtained for children, due to small sample size (fewer than 30 observations), and, therefore, are not reported.
Our analyses accounted for the complex sampling designs of the surveys. We used SAS, version 9.0, to estimate the weighted numbers and proportions of visits and their robust 95% confidence intervals (CIs). An exemption of review was approved by the University of North Carolina at Chapel Hill Institutional Review Board.
Discussion
This study analyzed data from NAMCS and NHAMCS to examine changes in the diagnosis of depression and its pharmacologic treatment in ambulatory settings before and after the FDA advisory on the risk of antidepressant-related suicidality among children and adolescents. The data source provided a unique opportunity to examine providers' prescribing behavior at a national level.
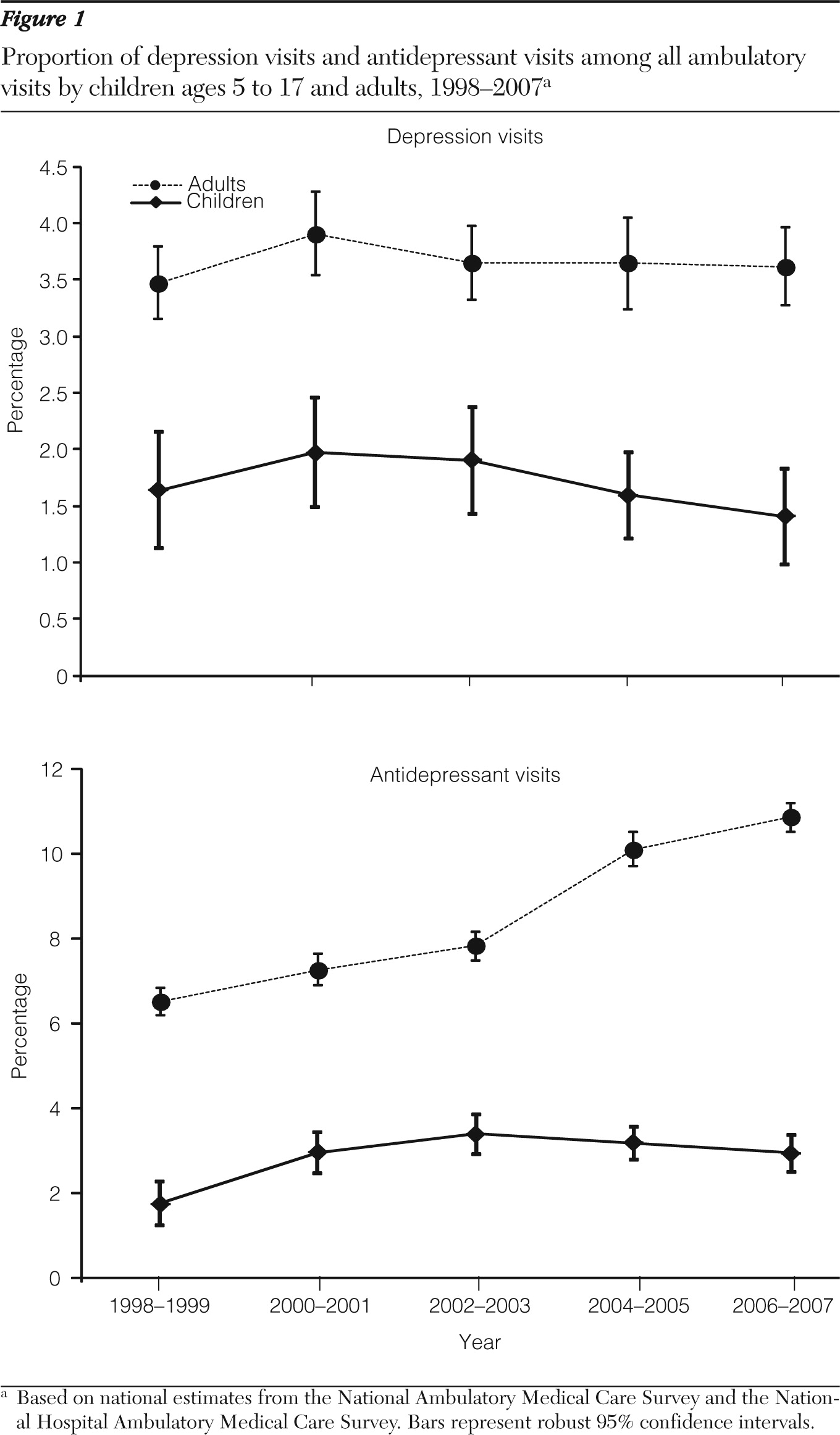
We observed a steady increase in the number of ambulatory visits with a diagnosis of depression among children from 1998–1999 to 2002–2003. After a series of FDA advisories, in 2003 and 2004, the upward trend was interrupted, and by 2006–2007 the number was similar to what it had been in 1998–1999. Our findings are consistent with previous studies that examined the frequency of monitoring for depression using longitudinal patient-level data (
8,
9,
13). An apparent decrease in visits with a diagnosis of depression, which could not be explained by changes in the number of all visits made by children, might have been an unexpected and unintended consequence of the FDA advisory. One would expect more frequent monitoring of depressed children treated with antidepressants as a result of the advisory, possibly leading to a higher number of visits. Unfortunately, the data did not allow us to determine whether the decrease was due to new depression cases being identified less frequently, less frequent follow-up of existing depression, or a combination of both.
Changes in the behaviors of providers, patients, and caregivers after the advisory was issued might at least partly explain our findings. Little is known about how physicians reacted to the advisory. Studies using pharmacy dispensing records may have estimated a mixture of changes in both provider behavior (to prescribe antidepressant) and patient behavior (to fill an antidepressant prescription). On the other hand, using NAMCS and NHAMCS data allowed us to capture the overall trend of prescribing antidepressants for pediatric depression.
Mental health diagnoses are often subject to undercoding due to fear of stigma (
19). In clinical practice, diagnoses are often tied to treatment plan. It is possible that the decrease in physicians' diagnosis of depression reflected the narrowing scope of antidepressant use after the advisory. Unfortunately, data from NAMCS and NHAMCS do not reveal how health-seeking behaviors among pediatric patients with depression and their caregivers might have changed after they were exposed to information about suicidality risk of antidepressants.
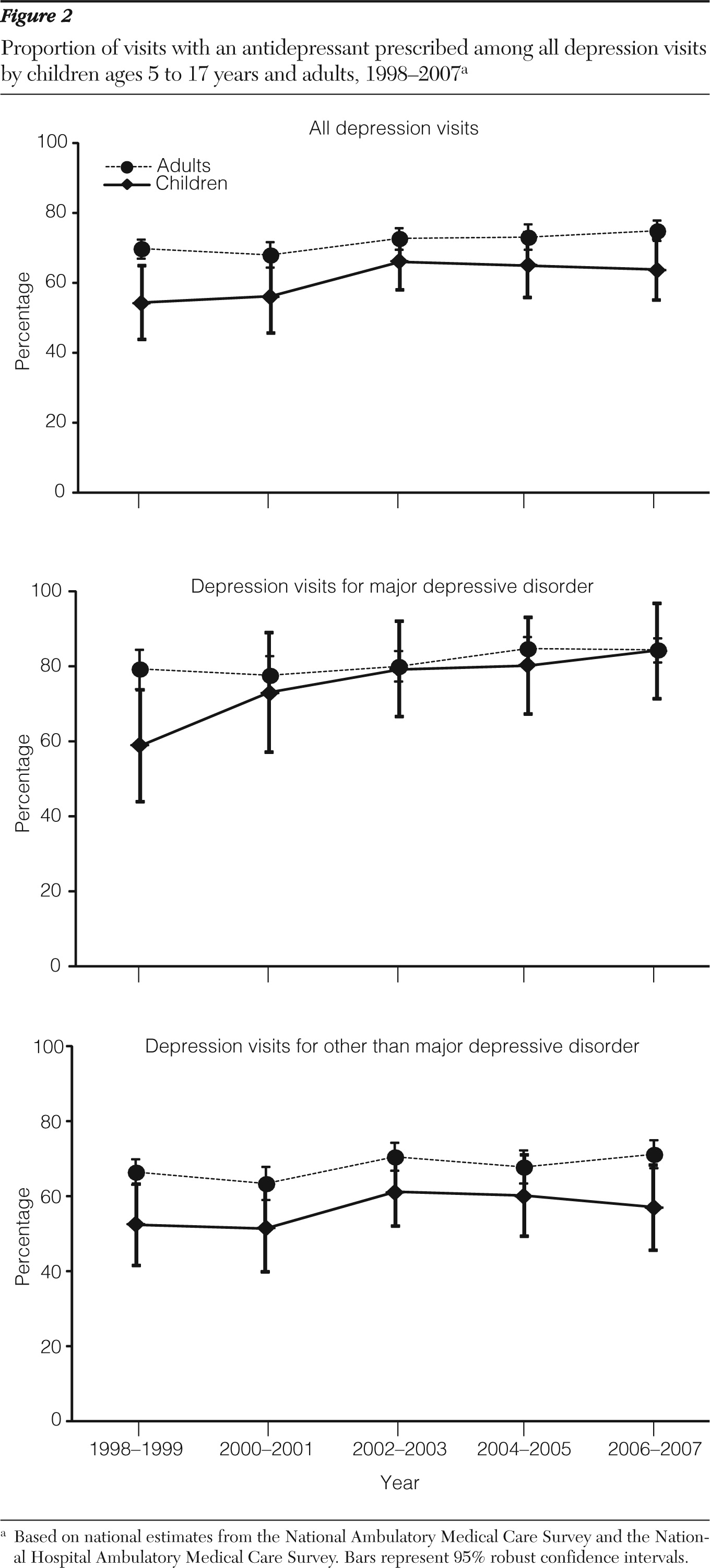
As found in prior studies (
6–
8,
10,
11), we observed a decline among children in the number of depression visits with an antidepressant prescribed after the FDA advisory, a trend that seemed to parallel an overall decrease in depression visits. When we further assessed the trend in terms of proportion, the likelihood of prescribing an antidepressant among visits with a diagnosis of depression among children remained relatively stable at approximately 65% just before and after the advisory. A similar trend in visits to psychiatrists was found when analyzing NAMCS data. These findings suggest that the FDA advisory might have led to fewer numbers of depression visits overall and of depression visits with an antidepressant prescribed. But even with this decline, if children were actually diagnosed as having depression, they might not have been any less likely to have been prescribed an antidepressant after the advisory than they had been before it.
This stable trend appeared to be a result of an increase in prescribing antidepressants in visits for major depressive disorder and a decrease in prescribing them in visits for other depression. This pattern could be an indication that providers responded to the advisory differently on the basis of the severity of depression or a change in coding practice after the advisory. In their recent analysis of administrative claims data, Valluri and colleagues (
20) also found a decreased likelihood of receiving antidepressant treatment for youths overall but not for those with major depressive disorder.
Prior studies have reported decreased use of SSRIs among children since the advisory was issued (
5,
7,
8,
10), but only one study that used pharmacy dispensing data reported findings separately for fluoxetine and other SSRIs (
6). Our results are consistent with the findings of decreased use of other SSRIs but an uptake in use of fluoxetine. On the other hand, we did not observe an effect of the FDA advisory on the utilization patterns of specific antidepressants among adults.
The study had several limitations that should be acknowledged. First, we could not determine the exact moment in time when the impact of the FDA advisory might have occurred. Several regulatory actions were taken by FDA in 2003 and 2004, but we used the advisory in 2003 to divide the study period into preadvisory and postadvisory periods. The impact of the advisory and other actions might not have been immediate and could have affected organizations, health care providers, patients, and caregivers at different rates. Because these actions took place over a period of time, we were not able to disentangle the impact of specific actions and might not have allowed for a reasonable lag time between the regulatory actions and manifestation of their impacts.
Second, only two data points (2004–2005 and 2006–2007) after the FDA issued its advisory were available for analysis. Data from more recent years, once available, should be analyzed to determine the longer-term impacts of the advisory and other FDA actions on changes in both antidepressant use and the incidence of suicidality.
Third, the use of data from a cross-sectional, encounter-based survey did not allow for longitudinal follow-up of unique patients. We could not determine whether a visit was made by a patient with a new or an existing episode of depression, nor could we differentiate antidepressant visits by initiation, continuation, or switching of medication. NAMCS and NHAMCS provide an annual “snapshot” of ambulatory care in the United States. To examine the impact of the FDA advisory on changes in the management of new or recurrent episodes of depression, and on antidepressant initiation or continuation, one needs to study a well-defined population whose medical and treatment history are available over time. Additionally, the data used in this study did not include other providers that care for children with depression, such as social workers or therapists.
Fourth, our findings should be interpreted in the context of overlapping confidence intervals. Small sample size also precluded us from analyzing data about young adults between 18 and 24 years old. Finally, our study examined the overall trends in utilization and could not assess the appropriateness of care or evaluate its impact on clinical or public health outcomes.