The majority of behavioral health care in the United States is delivered in primary care settings (
1). Low socioeconomic status is associated with increased rates of behavioral health problems, and safety-net primary care providers are increasingly providing treatment for patients with depression, bipolar disorder, substance use disorders, and suicide risk (
2,
3). Development of a systematic approach to depression treatment in primary care has been identified as a quality improvement target by the Surgeon General, and although there is a strong evidence base for primary care-based depression treatment (
4), substantial barriers remain to implementation of such programs.
An ideal health care system would include fully integrated behavioral and general medical services, providing treatment in primary care when appropriate and seamless collaboration with specialty care when needed. However, community health centers (CHCs) and community mental health centers (CMHCs) are separated by distinct reimbursement and regulatory structures. The division of behavioral health and general medical health is particularly problematic for people with severe mental illness, in part because of the impact of poor health habits, such as smoking, and long-term effects of antipsychotic medications on general medical health—for example, metabolic syndrome. Most CMHCs are not equipped to support medical management of metabolic syndrome (
2,
5).
Diffusion of innovations is a long-standing challenge that limits the general public's benefit from quality improvement advances (
6). The learning collaborative is a well-established framework for the spread of evidence-based treatments of chronic conditions, including behavioral health problems (
7–
9). Learning collaboratives teach and use a variety of quality improvement skills and methodologies. Perhaps the most popular model of learning collaborative is the “Breakthrough Series” developed by the Institute for Healthcare Improvement (
10). Key elements of the Breakthrough Learning Collaborative include selecting a change target, recruiting expert faculty, developing a framework, enrolling participants, initiating preparation for change, implementing learning sessions interspersed with work periods, and holding a final learning congress. Of particular note is the emphasis on a structured approach to the change process, namely the rapid cycle improvement (RCI) model of incremental process change (
10). A complete description of the history and application of learning collaboratives is available from the Institute for Healthcare Improvement (
10).
Learning collaboratives have demonstrated effectiveness for a variety of health services goals (
7), but data appear to be limited on whether the learning collaborative model ensures improvement in patient-level outcomes when applied to chronic illnesses (
11,
12). Nevertheless, it is a principal quality improvement approach used in CHCs (
7).
In this article we describe a large learning collaborative initiative sponsored by the National Council for Community Behavioral Healthcare (National Council) aimed at promoting collaboration between CHCs and CMHCs and with the goal of improving treatment of depression and bipolar disorder in CHCs and improving care of patients at risk of metabolic syndrome in CMHCs.
Methods
National Council's project
Specific goals for the National Council's Primary Care-Mental Health Collaborative Care Project were to increase the ability of CHCs to identify bipolar disorder, substance use disorders, and suicide risk as part of screening and care for depression and to increase the capacity of CHCs to provide follow-up and management of primary care patients identified as having depression. Increasing the integration of services between CHCs and CHMCs was also a goal of the initiative: namely, to increase CMHCs' provision of psychiatry training and clinical support for CHCs and to establish processes for collaboration between CHCs and CMHCs, including implementing protocols for referral of CHC patients with bipolar disorders or suicide risk to CMHCs, supporting the return of stable patients to primary care, and establishing methods for shared medical management of CMHC clients at risk of metabolic syndrome. Another important goal was to increase the capacity of CHCs and CMHCs to track care processes and performance.
The learning collaborative series
From 2007 to 2009, three separate cohorts were supported in a one-year learning collaborative. A total of 112 pairs of CHCs and CMHCs with preexisting collaborative relationships applied to participate in a competitive process. Sixteen pairs were selected to participate (four in the first cohort, eight in the second, and four in the third). The participating teams were given a small stipend (under $10,000) to support implementation and travel expenses for each site's team to attend an initial learning session and a final learning congress. Faculty members were experts in collaborative care for depression, management of bipolar disorder, detection and management of patients at risk of metabolic syndrome, and quality improvement. Participating teams attended an initial two-day learning session, participated in quarterly telephone calls to share progress, and attended a two-day final learning congress after 12 months.
Although the basic goals for each cohort were the same, the process was modified for successive phases on the basis of the experience of National Council staff and faculty and the feedback provided by participants in the earlier phases.
Measurement-based practice
Effective chronic care models promote the use of standardized measurement of treatment progress and disease registries to guide treatment. Systematic measurement of key clinical processes and outcomes was also a key component of the RCI change method promoted in this learning collaborative.
Measures
A requirement for participation in the learning collaborative was that CHCs were already conducting depression screening with the nine-item version of the Patient Health Questionnaire (PHQ-9) (
13). It was an explicit goal to extend the use of the PHQ-9 beyond case identification to include tracking of treatment progress and to inform treatment changes.
In the learning collaborative, the goals for bipolar disorder, substance use disorders, and suicide risk management were primarily to identify cases and initiate treatment; thus we focused on the use of screening tools for these conditions. For bipolar disorder, we supported the use of either the Mood Disorder Questionnaire (
14) or the bipolar section of the World Health Organization's Composite International Diagnostic Interview, version 3 (
15). For alcohol use disorders, we supported a composite of the first three items from the Alcohol Use Disorders Identification Test Consumption (
16) and the CAGE-AID (
17). For suicide risk, we supported the use of the Suicidal Behaviors Questionnaire-Revised (
18). We utilized a set of RCI metrics developed by the Standards for Bipolar Excellence Project (STABLE) that are aimed at improving recognition and evidence-based management of bipolar disorder (
19). We augmented the STABLE metrics with depression-specific quality measures. [A chart with detailed information about the metrics is available in an online appendix to this article at
ps.psychiatryonline.org.]
Self-assessment
Each participating site was asked to complete an initial self-assessment focused on the specific goals of the project. Starting with cohort 2, we used the Assessment of Primary Care Resources and Supports for Chronic Disease Self-Management (PCRS), which was developed for the Robert Wood Johnson Foundation Diabetes Initiative (
20). The PCRS focuses on patient support and organizational support, each of which has eight distinct elements to be scored. Scoring is based on four levels of performance, each with narrative anchors. The CHCs and CMHCs were asked to focus on two separate areas. CHCs focused on self-management of depression, bipolar disorder, substance use, anxiety, and other behavioral health conditions. The CMHCs focused on self-management of blood pressure, lipid levels, obesity, and glucose levels and diabetes, as well as other chronic health conditions found in the population served. The PCRS was used as a postassessment tool for cohort 2, whereas for cohort 3 we used the PCRS for both pre- and postassessment.
Participants
Sites were recruited through membership channels of the National Council. The selection process was conducted by the learning collaborative faculty and National Council staff. Selection was prioritized on the basis of the strength of the established relationship between the CHC and CMHC; whether depression screening with the PHQ-9 was currently implemented; and for representation of urban, rural, and frontier teams.
The learning collaborative
From the perspective of participating sites, the learning collaborative consisted of the following components: application, self-assessment, an initial learning session, RCI activities, interim reports (
3), quarterly conference calls with faculty, a final report, and a final learning congress.
In the application, teams were asked to describe the populations they served, the nature of their existing collaboration, and shared goals for the project. After selection and before the initial learning session, teams were asked to work together to complete the PCRS. At the learning session, the teams in each cohort were brought together for detailed briefings on the project and presentations by content experts. The initial one-day learning session for cohort 1 was extended to two days for cohorts 2 and 3 to allow teams to begin developing RCI (PDSA—plan, do, study, act) plans with direct input from faculty.
At the learning session, sites were asked to select at least ten of 15 RCI metrics and to specify an achievement goal for each measure. For the initial, interim, and final reports, teams were asked to complete preformatted report forms that included PDSA worksheets and a template for documenting progress. The template included numerator and denominator specification for each of the performance measures. After the initial learning session, teams were asked to collect baseline data for a brief period (one to two months) before initiating practice changes.
Reports were analyzed by faculty, and quarterly conference calls were held to provide feedback to sites. The conference calls were held collectively for cohort 1 and then more individually for the teams participating in cohorts 2 and 3. Although this came at the expense of shared learning, the shift was deemed necessary because the teams were so diverse in terms of their focus and support needs.
Sites then entered the practice change phase. The interim report included postbaseline data and current PDSA plans. Again conference calls were held to address implementation issues. This process was repeated a third time. Before the final learning congress, sites provided their most recent PDSA worksheets, RCI metrics for the period, and summary reports of overall progress, barriers, and lessons learned.
The collaborative ended with a two-day final learning congress in which all teams in the cohort presented their results. Faculty summarized progress across all teams.
Results
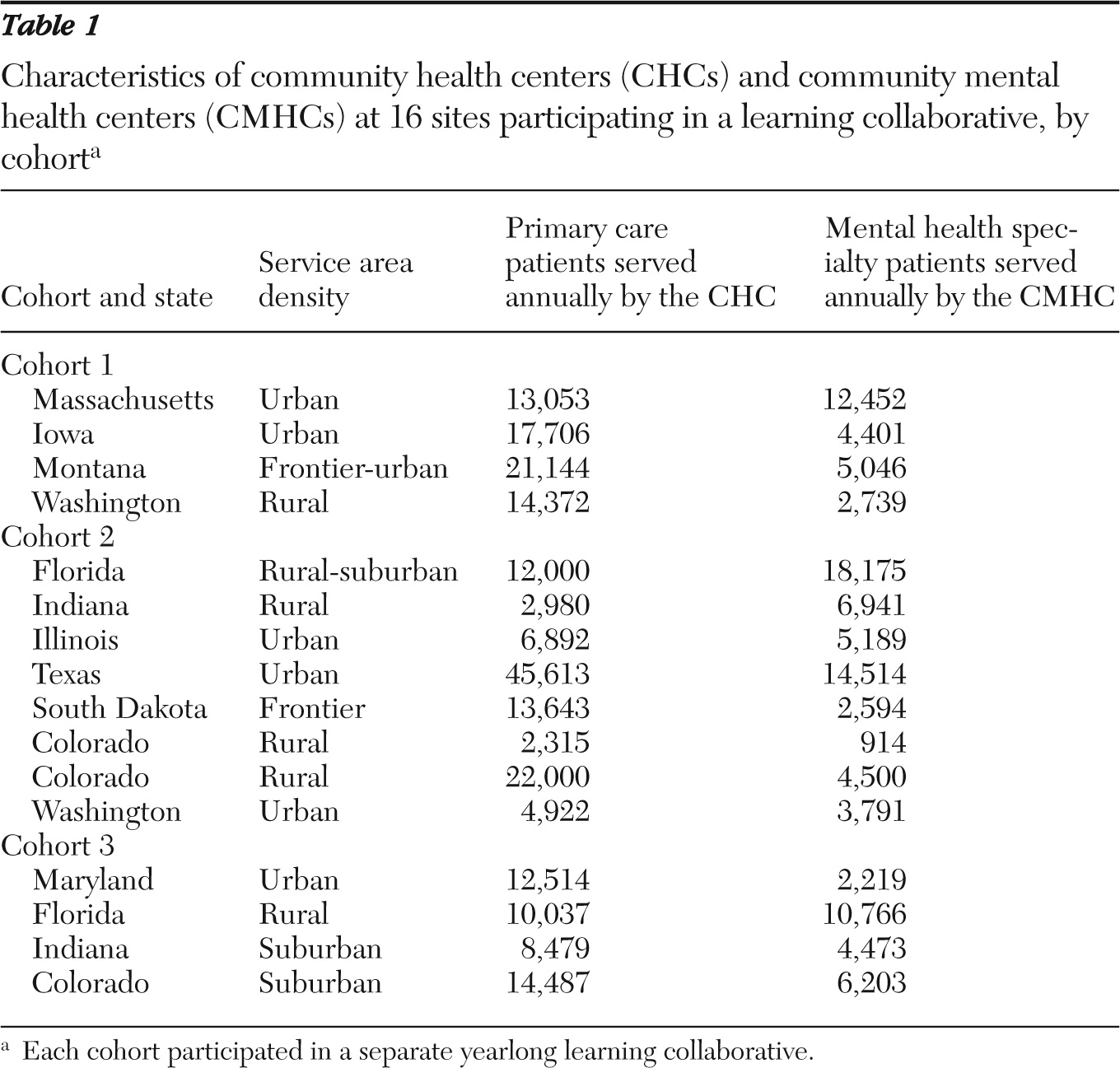
As shown in
Table 1, the 16 sites selected for participation represented 11 states. One team in cohort 2 dropped out of the project. These sites had already implemented screening for depression (a precondition for selection); thus the teams could choose from the 14 remaining RCI metrics (see online appendix at
ps.psychiatryonline.org).
Tracking of specific measures varied between sites. One-time measures, such as screening or administration of the PHQ-9 at the time of diagnosis, were most common, whereas measures that were longitudinal were used less frequently. Registries are a logical resource for measurement-based practice. A few of the participating teams were able to create registry capacity, but most found it challenging to consistently generate, track, and report key measures such as PHQ-9 depression scores. It was most important for teams to track their own change over time as they implemented the RCI process. Comparison across teams on specific metrics turned out to be impossible because of differences in how each site implemented its improvement and tracking strategies (for example, one site elected to track a subsample of its patient population that was seen by a specific provider, whereas another elected to track across providers all diabetic patients with depression).
Self-assessments
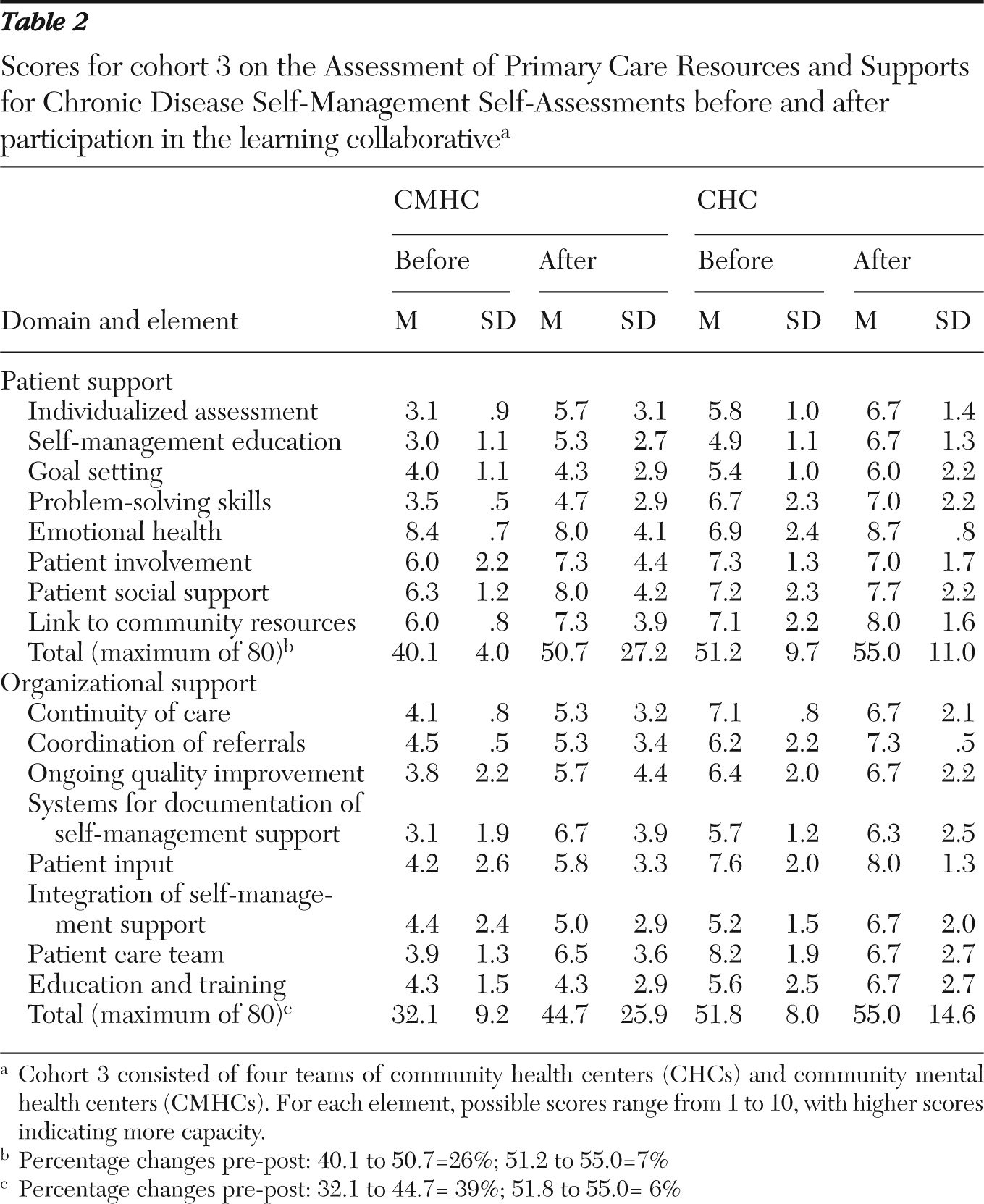
The total number of possible points for patient support in the PCRS is 80. For the 16 sites, the mean±SD preproject score for the CHCs (51.2±9.7, range 39–60) was higher than the score for the CMHCs (40.1±4.0, range 36–45). The total number of possible points for organizational support in the PCRS is 80. The CHCs' mean preproject score (51.8±8.0, range 45–48) was almost 20 points higher than the CMHCs' score (32.1±9.2, range 24–43). A possible explanation for the CHCs' higher self-reported scores is that most of the participating CHCs had prior experience with RCI and with application of the chronic disease model through their participation in the Health Disparities Collaboratives supported by the Health Resources and Services Administration. Results on the PCRS self-assessments for cohort 3 are provided in
Table 2.
Compared with CMHCs, CHCs had higher self-ratings of both patient support and organizational support before and after participation in the learning collaborative. CHCs' initial ratings were higher than the CMHCs' final ratings. However, the CMHCs showed greater increases in these ratings than did the CHCs (11.5% and 5%, respectively).
Participant satisfaction
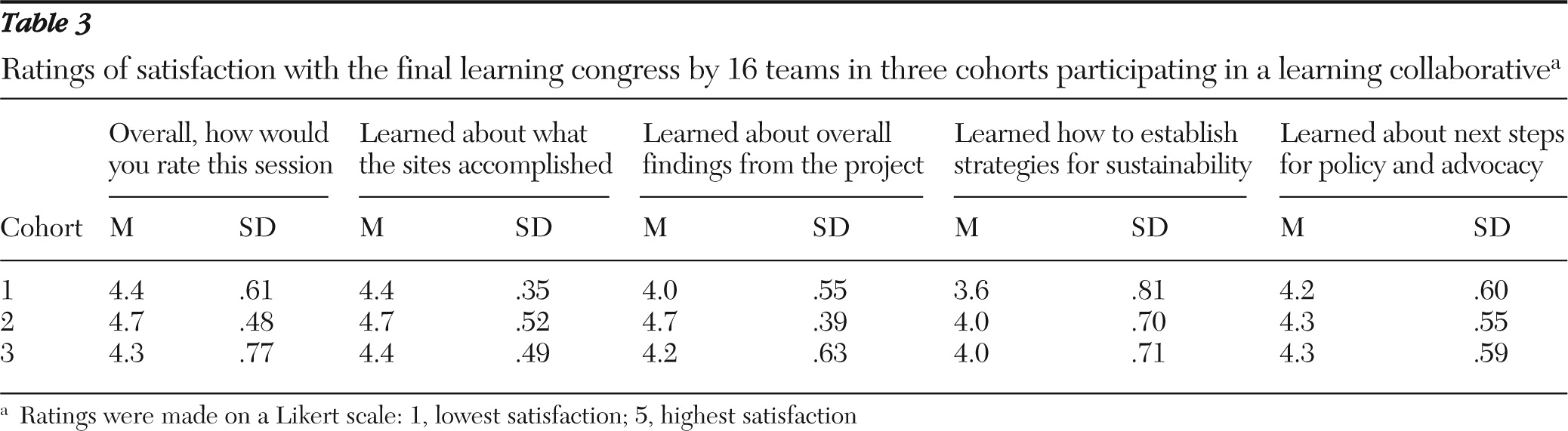
Teams rated their satisfaction with the final learning congress, and satisfaction scores are presented in
Table 3. The mean rating was high on all five items (4.3±.3).
Process observations
Successful RCI efforts require careful attention to operational details and workflow. One CHC, for example, attempted to reach 100% screening for all patients by having the instruments handed out during patient check-in. After all the initial screening forms had been used up, several weeks went by with no screening. When performance data were reviewed, it was discovered that screening had stopped because nobody had been tasked with restocking the forms.
Turnover is also a major threat to successful practice change efforts. This was particularly problematic for staff who were trained in care management tasks because this training is available only through postgraduate seminars and workshops, which made it difficult to replace staff who vacated this key role on the integrated care teams.
We had hoped that the focus on integration of behavioral and general medical services through strengthening existing relationships between CHCs and CMHCs would lead to the establishment of more primary care services in CMHCs. However, this occurred for only one team during the collaborative; a primary care physician was placed one day per week in a CMHC to help clients with severe mental disorders and unmet general medical needs.
All teams found it challenging to establish effective methods for ongoing collaboration and communication. Teams reporting successful collaboration often noted specific ways in which team members and other personnel involved had initiated and maintained regular communication. Usually such communication included regular, structured meetings, especially early in the project.
Substantial and exciting changes in clinical practice occurred at each of the participating sites, and these changes differed widely, reflecting differences in needs and capacities at the sites. To illustrate the changes made, we describe in the online appendix the experience of one of our participating teams, a CMHC and a CHC in a suburban Midwest setting (
ps.psychiatryonline.org).
Discussion
The National Council's Primary Care-Mental Health Collaborative Care Project sought to increase the capacity of CHCs to deliver evidence-based depression treatment; to identify patients with bipolar disorder, with alcohol use disorders, and at elevated risk of suicide; and to attend to the metabolic side effects of antipsychotic medications.
All of the teams that participated in this learning collaborative presented evidence at the final learning session (and throughout the learning collaborative) that they had made progress toward implementing some portion of the quality improvement targets outlined in the project. No team was able to make clear progress on all targets. Below we discuss several issues that teams and faculty identified as barriers to the quality improvement efforts.
Lessons learned
The implementation of bidirectional integrated care (behavioral health screening and intervention in primary care settings and screening and management of common medical problems in specialty settings) is a daunting challenge, as documented in this learning collaborative and in other initiatives. Inability to track patient outcomes and analyze data at both the patient and system levels remains a substantial barrier to measurement-based practice. Even sites with well-established electronic health records struggled with this challenge.
Teams that established effective communication and problem-solving strategies showed more progress. Integrated care remains difficult to finance. The variability in state Medicaid systems, particularly for CMHCs, made problem solving very much a local effort. Some teams actively advocated at the state level for better financial support of integrated care, with promising results. The new structures and financing methods in health care reform (such as medical homes, accountable care organizations, case rates, and bundled payments) offer opportunities to address financing barriers to integrated care. However, overcoming the financing barriers will require dedicated attention and advocacy at both national and state levels.
Participating sites enjoyed and made good use of the opportunities to learn from each other, but cross-site learning promoted in the learning collaborative model can be difficult to realize when sites are not starting at the same place.
Didactic presentations at learning sessions are necessary, but they should be kept short and to the point. Conversely, substantial time should be devoted to work sessions in which participants receive consultation from faculty on specific clinical workflows and PDSA cycles and share their experiences with other participants.
Four to six teams may be an optimal cohort size. Larger cohorts may make it difficult to develop individual relationships with faculty and other teams.
Because all practice change is local, it is difficult to develop a general structured process that fits all participants. However, specific tools were strongly welcomed, such as PDSA worksheets, Gantt charts, referral forms, and screeners. These tools were freely shared between faculty and participating teams and were considered extremely helpful by all teams.
Measuring participant satisfaction and skill development along with phased implementation provided valuable feedback to the learning collaborative process itself, allowing for dynamic quality improvement on an ongoing basis.
What we would do differently next time
It is often too challenging for a team to work simultaneously on improving behavioral health care in primary care and primary care in behavioral health care settings. After participating in the learning collaborative, several participating teams went on to develop improved primary care in their partnering CMHC. A sequential learning collaborative that builds on early successes in developing communication and collaboration might be more effective than attempting to achieve improvement in both settings simultaneously.
In future efforts, we would provide more concrete tools and spend more faculty time focused on team communication. As with data entry, this can be perceived as “one more meeting that I have to go to”; however, teams that mastered these tasks were more satisfied with their progress and closer to reaching the overall project goals.
Conclusions
All participating sites made practice changes that required ongoing organizational supports and use of measurement to sustain the improvements. A successful learning collaborative must assist teams in breaking the change process down into clear operational strategies and focusing attention on detailed shared clinical workflows. Role clarification is a key to successful practice change. Our experience with supporting the integration of care in this learning collaborative is consistent with similar observations by Nutting and colleagues (
21–
23), who studied a two-year medical home practice transformation project. Integrated mental health care requires system transformation and substantial practice change. It also requires substantial commitment and transformation of participating primary care and mental health providers, who must develop truly integrated and shared workflows and effective communication and collaboration. Technology needed for such integration is not “plug and play.” “Change fatigue” and competing initiatives are challenges even for the most committed practices. Separate financing systems continue to be a major real or perceived barrier to effective integration of services.
To reach the desired goals, all of the required “transformations” occur in the context of diverse local resources and relationships. Given the substantial overlap of the changes needed to implement integrated mental health care and patient-centered health care homes, we hope that these efforts can be woven together as we advance an agenda of cost-effective patient-centered integrated care.
Acknowledgments and disclosures
The authors thank the 16 teams that participated in the three phases of the learning collaborative. The CMHC in the case study presented in the online appendix is Regional Mental Health Center in Merrillville, Indiana. The CHC was NorthShore Health Centers in Portage, Indiana.
Dr. Vannoy, Dr. Unützer, and Ms. Mauer received payment as faculty consultants for this project from the National Council. The other authors report no competing interests.