Typically, such studies are based on quantitative, volumetric measurement of brain structures within individual subjects, a laborious task that entails generating specific anatomical structures, or regions of interest. This may be accomplished by a variety of methods. Regions of interest that have boundaries where there are marked differences in signal intensity, such as ventricles, are suitable for automated techniques such as edge-finding and selection of pixels by signal intensity parameters (“thresholding”). For other brain regions that have poorly defined borders (in terms of signal contrast), automated methods for defining regions of interest are less accurate. Demarcation of regions of interest in such areas often requires manual outlining and neuroanatomical expertise.
The fact that intersubject averaging has not been widely used in structural MR image analyses despite these advantages may relate to the greater intuitive difficulty in interpreting differences in the signal intensity measure underlying structural MR compared to functional imaging. Functional imaging is quantitative along a continuum of a given measure or index of cerebral activity, with different data values reflecting proportional differences in the amount of cerebral activity. In contrast, differences between MR data values may connote a categorical difference (e.g., CSF versus gray versus white matter), variations in underlying chemical properties, or scanner-related nonuniformity artifacts. These differences, for which the meaning is dependent on magnitude and location along the signal intensity spectrum, are more difficult to understand. The quantitative interpretation of MR data—as opposed to positron emission tomography (PET) or computed tomography imaging—is further complicated by the lack of absolute signal normalization (i.e., any fixed scale on which signal intensity is based).
We now report the results of an image averaging analysis of MR scans obtained from 25 schizophrenic and 25 normal control subjects as part of a larger MR study in schizophrenia. The goals of this study were to further evaluate the validity of linear image averaging methods in structural analysis, to examine potential artifacts that might be introduced by this method, and to expand the anatomic extent of our survey of differences between schizophrenic and normal subjects. Since we also specifically wished to further assess evidence for thalamic structural abnormalities in schizophrenia, the method used here attempted to reduce misregistration in central brain.
METHOD
Twenty-five schizophrenic and 25 normal control subjects (all men) were selected for the present analyses from a larger MR study sample of 72 schizophrenic patients and 29 control subjects. All subjects were given a complete study description before written informed consent was obtained. Subjects were recruited from a Veterans Affairs hospital in the metropolitan New York area. Schizophrenic patients were recruited from inpatient and outpatient hospital facilities. Schizophrenic subjects were diagnosed by two psychiatrists and met DSM-III-R criteria for schizophrenia on the basis of information collected with the Structured Clinical Interview for DSM-III-R—Patient Version (
40). Normal control candidates responded to a posted hospital announcement and were screened initially by telephone before receiving an evaluation interview (
41). The subgroup of 50 subjects reported here was selected, without any prior knowledge of MR results, from the overall sample to match for age and parental education.
General exclusion criteria for all subjects included age over 50; any contraindication to MR; current medical illness (including hypertension); history of head trauma, loss of consciousness, or seizures; mental retardation; neurological disease or CNS infections; or ongoing substance abuse. One schizophrenic patient had a history of prior substance (alcohol) abuse. Normal control subjects were also excluded if they had a first-degree relative with a history of psychiatric illness, an education level greater than 16 years, or any current or past DSM-III-R psychiatric diagnosis based on the SCID-NP (
42). All scans were assessed initially by a clinical radiologist, and subjects with scans showing gross brain pathology (i.e., other than mild atrophy or ventricular enlargement) were also excluded from the analyses.
Medical history, medication history, a handedness inventory (
43), and demographic information were obtained from all subjects. Clinical ratings, including the Brief Psychiatric Rating Scale (BPRS), Schedule for Assessment of Negative Symptoms (SANS), and Clinical Global Impression (CGI), were obtained at a time point thought to represent maximal clinical stability relative to the patient's current episode; this time ranged from the day of the MR scan to 12 weeks after the scan. All schizophrenic subjects were receiving neuroleptic treatment at the time of their MR scan.
High-resolution MR images were acquired on a 1.5-T scanner (Picker Vista HPQ). The imaging protocol started with the acquisition of a sagittal scout sequence. The orientation of the coronal plane was determined from the midsagittal scout image as perpendicular to the line connecting the bridge of the nose and the base of the occipital lobe. This was done to minimize variability in patient positioning. The scout sequence was followed with two clinical sequences yielding proton density-weighted and T2-weighted axial images. A high-resolution, three-dimensional gradient echo sequence was acquired coronally and provided the T1-weighed images used in the present analysis. In a preliminary study we have optimized this gradient echo sequence to achieve maximum gray matter/white matter and CSF/gray matter contrast. The selected parameters were as follows: TR=33 msec, TE=11 msec, flip angle=35 degrees, 256×256 acquisition matrix, slice thickness=2.8 mm, and one signal average.
Images were transferred to a local work station (Sun Microsystems), were measured for angles and coordinates through use of a locally developed C-language program (MIDAS) (
44), and were transformed by using locally developed C shell script batch jobs. The three basic image transformation steps included 1) reangulation, 2) linear stretching, and 3) gray level normalization. Full details of the image analysis are described in appendix 1. In brief, methods were as follows.
Images were first reangulated in two successive steps so that the plane defined by the interhemispheric fissure corresponded to the sagittal plane of the coordinate system. A final reangulation was performed to orient slices perpendicular to the rostrocaudal dimension at a 21-degree angle relative to the slope of the line connecting anterior and posterior commissures, equal to the overall average orientation from the anterior commissure and posterior commissure line for the entire sample. (This orientation, instead of the true anterior-posterior commissure plane of the Talairach system, was used in order to minimize potential interpolation artifacts caused by large angle rotation.)
Next, through use of MIDAS (
44) software that permitted scrolling through the various slices of the brain, a series of redundant measurements were obtained that defined six coordinates representing the extreme points on the brain surface in all three dimensions. In addition, coordinates were ascertained for the midpoint of the segment connecting the anterior and posterior commissures.
These values were then averaged for the entire set of 50 scans. All brain scans were then repositioned relative to the coordinate system so that the midpoint of the segment connecting the anterior and posterior commissures was in the same location for all subjects. Finally, through use of trilinear interpolation methods, the eight octants of the brain were linearly scaled to align their extreme points without disturbing the location of the midpoint. Thus, at the end of this step, all the variance due to global extent of the cortex in each of the six directions was eliminated.
Since MR signal intensity is not calibrated across scans, image uniformity correction was performed by using white matter signal (determined by sampling regions of high confidence for tissue identification) as a reference. The means and standard deviations of signal intensity were then computed on a pixel-wise basis for each slice in the reformatted image set for both schizophrenic and control groups. This allowed generation of one-tailed effect size maps depicting pixel-wise differences in signal intensity for normal subjects minus schizophrenic subjects and for the opposite comparison of schizophrenic subjects minus normal subjects.
The planar alignment component of the image average method was examined by using interrater reliability analysis, which was based on 20 subjects being processed a second time by a different rater. For each case, the angle in the sagittal, axial, and coronal planes was measured separately by the two raters, and the absolute mean angular difference was computed. In addition, the absolute accuracy of the image averaging method itself was examined by an analysis of the error in positioning and linear stretching of images to a common coordinate space. This analysis excluded confounding variability arising from biological (nonlinear) differences in brain morphology. In brief, the coronal images from a single subject were tagged at 12 landmarks. Landmarks were selected on the basis of their relative ease of identification and so as to derive measures of positioning accuracy throughout a wide extend of the brain. (These are described further in
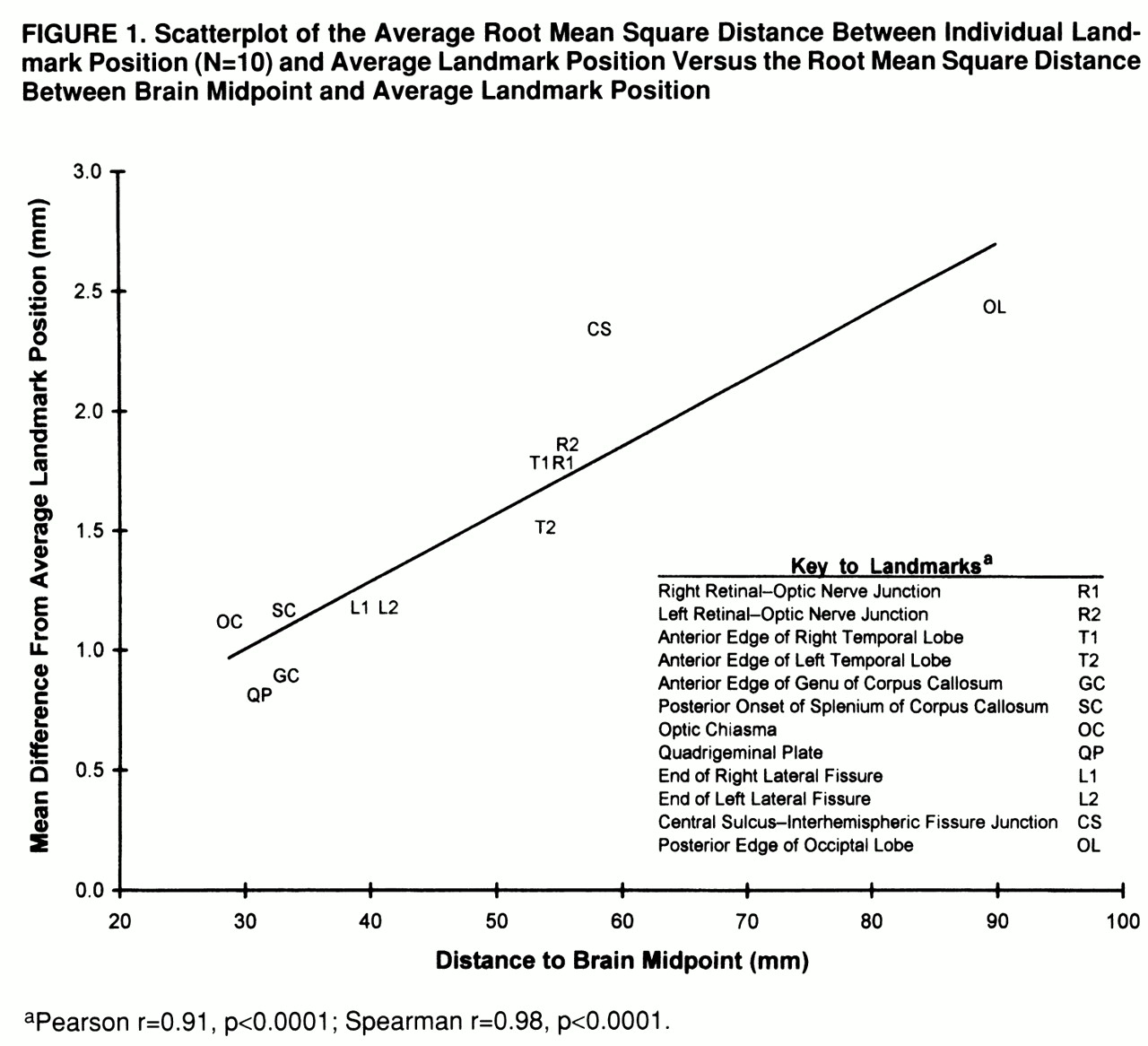
figure 1.) The images from this single case were then subjected to 10 different and random sets of linear image distortion and three-dimensional misalignment parameters, so as to create 10 sets of computer-generated phantom scans simulating differences in head size, orientation, and location within the scanner. The same coregistration and linear stretching method was then applied to each of these simulated cases. Ideally, this should have resulted in an identical placement of each of the 12 landmarks for all 10 cases. For each landmark, accuracy of coregistration was measured as the root mean square difference between the midpoint of landmark position of individual cases and the midpoint of average landmark position.
RESULTS
Schizophrenic (N=25) and control (N=25) subjects were closely matched for age (mean=38.0 years, SD=5.7, and mean=36.0 years, SD=8.9, respectively) (t=0.95, df=48, p=0.35). Although parents of schizophrenic patients had, on average, only 1 year less of education than parents of control subjects (mean=13.0 years, SD=1.1, versus mean=14.2 years, SD=1.8), this difference was statistically significant (t=2.54, df=46, p=0.02). Schizophrenic patients had a mean illness duration of 15.2 years (SD=6.6) and a mean age at onset of 22.8 years (SD=4.2), reflecting a moderately chronic population. Clinical variables also reflected a moderate severity of illness, despite neuroleptic treatment. The patients had a mean total BPRS score (0–6-point scale) of 23.1 (SD=7.9), a SANS global score (sum) of 12.2 (SD=3.9), and a mean CGI score of 4.1 (SD=0.8).
The interrater reliability analysis (N=20) of planar angle measurement for the two raters revealed a mean absolute angle difference of 0.6 degrees (SD=0.4) in the axial plane, 0.9 degrees (SD=0.5) in the coronal plane, and 1.4 degrees (SD=1.0) in the sagittal plane. The results of the coregistration validity study are shown in
figure 1. Overall, root mean square difference in positioning after coregistration ranged from 0.8 mm (SD=0.5) (quadrigeminal plate) to 2.4 mm (SD=1.3) (superior central sulcus) and averaged 1.5 mm (SD=0.5) for all landmarks. Furthermore, coregistration error directly correlated with the root mean square distance from the center of the brain (Spearman r=0.98, df=10, p<0.0001).
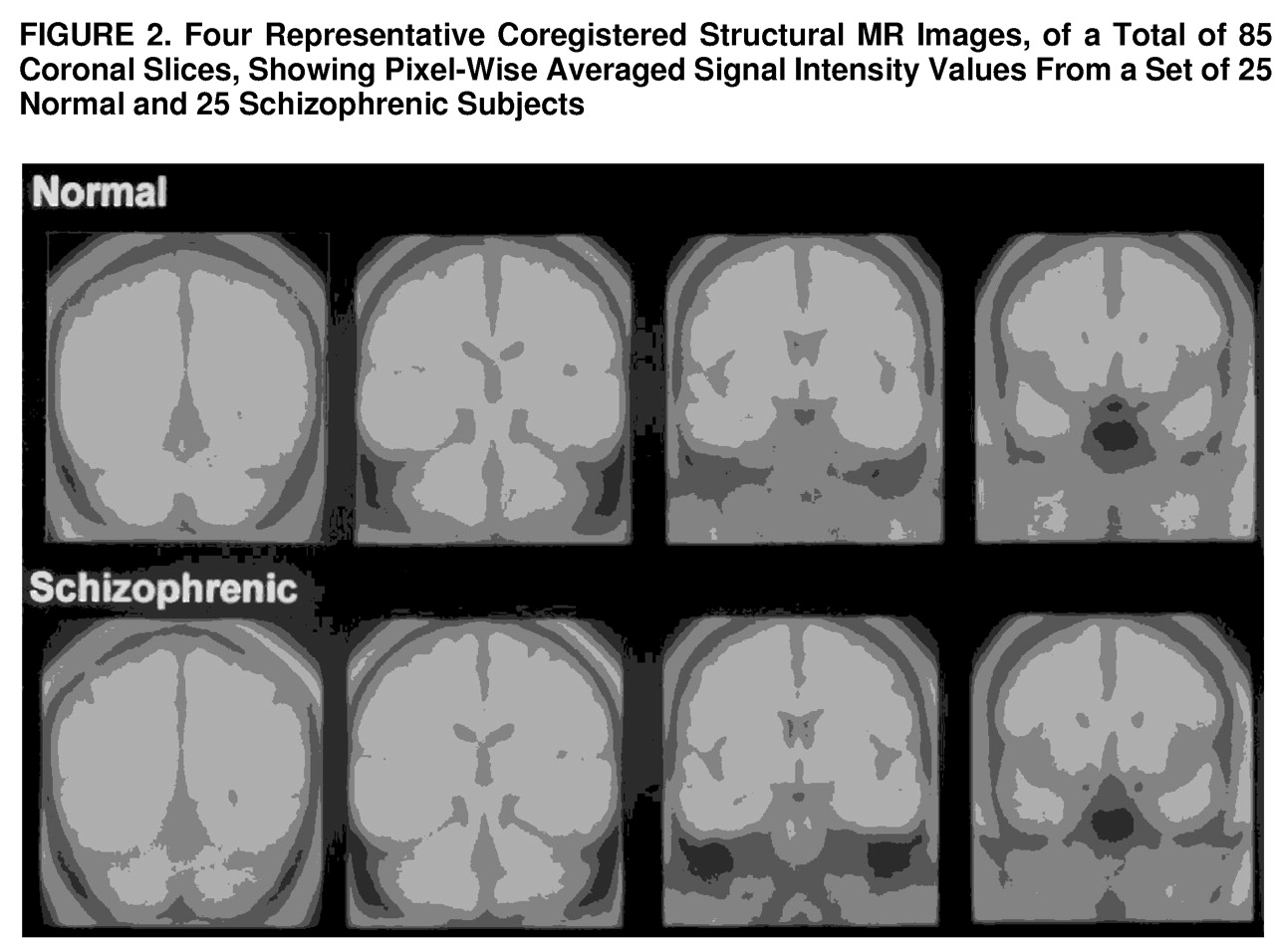
Averaged images for representative coronal slices are shown in
figure 2. As may be seen, there was excellent alignment along the anteroposterior dimension.
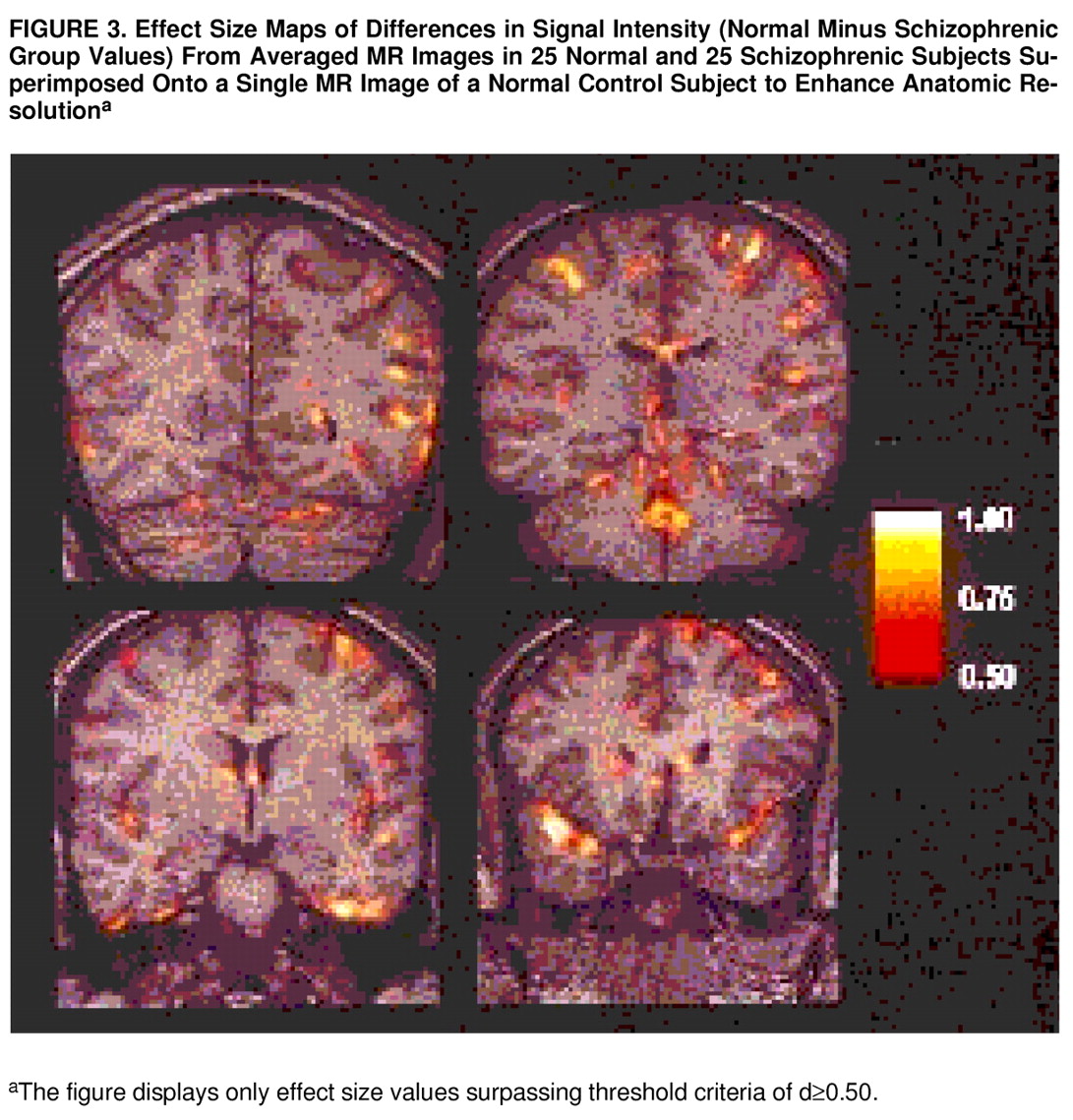
Effect size maps demonstrating one-tailed differences (values for normal group minus values for schizophrenic group) in signal intensity level are shown in
figure 3. These are four effect size maps out of a total of 85 averaged coronal images for which effect size maps were generated. The effect size maps are superimposed onto a single normal control case to enhance anatomic resolution. Effect size maps were thresholded to show only differences with an effect size ≥0.50, corresponding to a raw per-pixel one-tailed p value of ≤0.05. Examination of the groups of pixels with effect size ≥0.50 revealed a minimum cluster size of greater than 20 pixels per cluster. The likelihood of finding clusters of “active” pixels may be calculated theoretically by using the Euler characteristic of clustered sets (
45) or empirically by using Monte Carlo techniques as described by Roland et al. (
46), Poline and Mazoyer (
47), and Forman et al. (
48), who have generated probability tables for detecting active pixels as a function of cluster size and minimum per pixel alpha values. For a “raw” per-pixel alpha value of 0.05, clusters of 20 pixels or more correspond to a corrected false positive rate per pixel of <1 × 10
–6, indicating the strong statistical significance in signal intensity between normal and schizophrenic subjects in these areas.
Taken together, the entire set of images demonstrated rather diffuse signal intensity differences between normal and schizophrenic subjects in both cortical and periventricular areas. Periventricular differences were noted in the occipital tail, in the roof and medial aspects of the atrium, in the bodies of the lateral ventricles adjacent to both the corpus callosum and the fornix, in the frontal horns in areas adjacent to the caudate nucleus and also more rostrally adjacent to frontal white matter, in the fourth ventricle adjacent to the vermis of cerebellum, and around the third ventricle. (Because there were no group differences with respect to intrathalamic size and in lateral perithalamic white matter tracts, the signal intensity differences observed in the third ventricle suggest that abnormalities exist only at the thalamic-third ventricle interface and not in the thalamus itself.) Signal intensity differences in cortical areas were particularly prominent bilaterally in the middle occipital gyrus, bilaterally in the superior temporal gyri, in inferior and middle temporal gyri (more so in the left hemisphere), and in the left posterior parietal area. In addition, rather diffuse signal intensity differences were noted in frontal and prefrontal cortical areas, again more notably in the left hemisphere, particularly across the dorsolateral convexity and extending medially into the interhemispheric fissure.
Examination of effect size for the opposite tail, signal intensity for schizophrenic patients minus that for normal subjects, failed to reveal any pixels with an effect size greater than 0.25, and in almost all pixels it was much less.
DISCUSSION
Diffuse differences in signal intensity were found predominantly in periventricular and cortical areas at brain/CSF interfaces. These differences (indicating significantly lower signal intensity in the schizophrenic group) presumably represent the presence of CSF in place of brain parenchyma among schizophrenic subjects.
Cortical signal intensity differences were evident in the effect size maps as both sulcal enlargement and cortical thinning. The results are consistent with volumetric-based MRI reports demonstrating reduction in frontal matter (
11,
12,
15,
20–
22) or temporal lobe gray matter (
8–
12) or both. While volumetric deficits have been observed bilaterally in the temporal lobes (both cortical and subcortical), several studies have noted an asymmetric pattern (
16,
18,
49,
50), leading to the suggestion that temporal lobe gray matter deficit may be predominantly left-sided (
28). Two of the effect size maps included in
figure 2 suggest an asymmetric pattern of signal intensity differences. The coronal slice at the level of the thalamus demonstrates more extensive signal intensity differences in inferior aspects and less conspicuously in a subcortical region adjacent to temporal horn of the lateral ventricles (perhaps secondary to reduction in hippocampal extent). However, examination of a more rostral slice at the level of the frontal horns demonstrated very prominent signal intensity differences in the superior aspects of the temporal lobe, more so on the right. These data suggest that some of the discrepancy in asymmetry data from volumetric studies may derive from differences in the sites in which these measures were obtained.
The periventricular signal intensity differences—evident from the fourth ventricle through to the frontal horns—corroborate the consistent volumetric MR reports of ventricular enlargement in schizophrenia (
1–
7). They are also consistent with the results from our own volumetric analyses of ventricular regions of interest measured in the larger sample from which this group of schizophrenic patients was derived (M. Sanfilipo et al., unpublished data). It is of note that periventricular signal intensity differences were found not only adjacent to areas of subcortical gray, but also abutting prominent white matter structures (corpus callosum and fornix), as well as other diffuse white matter tracts (around the extremes of occipital and frontal horns, in particular). While a series of studies have reported reduction in size of the corpus callosum (
51–
53), there have generally been relatively few (
15,
54,
55) reports of white matter abnormalities in volumetric MR studies (although this tissue compartment has not been analyzed nearly as extensively as gray matter and CSF).
Taken together, these data are consistent with and extend the overall impression in the literature of diffuse structural abnormalities in schizophrenia (
12,
56–
62), at least in populations such as this. These findings highlight the challenge in ascertaining one (or more) pathophysiologic process that might account for such a pattern of structural abnormalities in schizophrenia. In particular, emphasis on cortical “lesions,” resulting either from aberrant migration (
63–
67) or neurodegeneration (
68–
70), appears inadequate in accounting for the prominent disruption of commissural and associational white matter tracts also seen here. Rather, these and other MR data, including Andreasen et al.'s image averaging report of perithalamic white matter deficits, provide grounds for speculation of a broader disruption in neural connectivity in schizophrenia.
In contrast to an image averaging study in schizophrenia by Andreasen et al. (
39), we did not observe any signal intensity reductions in the thalamus or in perithalamic white matter tracts. In addition, conversely, our findings of multiple areas of cortical and periventricular abnormalities were not apparent in their data (although the authors noted “subtle but visually detectable difference in ventricular size”). While signal intensity differences between schizophrenic and normal subjects in our study were extensive, they were limited to brain regions with a CSF-parenchyma interface. These areas are demarcated by a sharp gradient in signal intensity values. In contrast, the signal differences noted by Andreasen et al. occur in areas of relatively homogeneous signal intensity values. Both studies identified signal intensity differences in various white matter tracts.
Several factors might account for the different results from these two studies. First, there are clear methodological differences in the implementation of image averaging that limit direct comparison between the results of the two studies. As with all variations in image analysis techniques, each variation has its particular advantages and disadvantages. We normalized signal intensity across subjects on the basis of the signal intensity value corresponding to (individually—per slice sampled) values for white matter. We chose this approach, in part, because white matter regions offer high reliability for standardizing signal intensity. Further, this method does not entail manipulation of brain tissue (e.g., gray/white or gray/CSF) contrast that may be introduced by histogram normalization techniques, and it partially corrects for the imperfect receiver coil sensitivity of the scanner. Another major methodological difference lies in the stretching algorithm; we stretched each of the eight octants separately and maintained a fixed point in the brain, thus “correcting” for major asymmetries.
The differences in results might also relate to clinical differences between the two schizophrenic populations. The schizophrenic patients in our study had an average age of 38 years, had a mean illness duration of 15 years, and had psychiatric clinical ratings reflecting a moderate severity of illness; all of the patients had been receiving neuroleptic treatment for different durations and had different clinical responses. The schizophrenic sample studied by Andreasen et al. was somewhat younger (mean age=30 years) and had a markedly shorter duration of illness (mean=4.3 years). In addition, approximately one-fifth of their patients were neuroleptic naive. While ventricular enlargement is generally thought to be present by the clinical onset of schizophrenia (
71–
73), as the illness progresses there is evidence of additional enlargements, which are not accountable for by age (
69–
71). Similar data also exist demonstrating a more rapid progression of cortical atrophy at least in some schizophrenic patients than in normal control subjects (
74).
The sample size in our study had less power than the larger sample studied by Andreasen et al.; this theoretically raises the possibility of a type II error underlying the differences in results. However, the difference in average signal intensity throughout and around the thalamus (excluding periventricular regions) between normal and schizophrenic subjects was so minimal in our study that an increase from our sample size of 50 subjects to the number studied by Andreasen et al. (N=86) would result in only a trivial increase in power. For example, average signal intensities of the thalamus were 946 (SD=79) and 936 (SD=89) for schizophrenic and control subjects, respectively, yielding an effect size of 0.1 and a corresponding power of 0.10 (one-tailed p=0.05) (
75). Increasing the sample size to 86 would raise the power to only 0.12.
In more general terms, any image averaging method potentially introduces several sources of error. As discussed earlier in this article, proportionate techniques do not correct for nonlinear differences in brain morphology. The degree of this potential mismatching is subject to geometric and directional biases (
76); however, we are unaware of any systematic biases in regard to this effect that may have confounded results. In addition, the method introduces potential error in positioning and proportional alignment—what has been referred to as linear stereotaxy (
25). Coordinate-based morphometry systems such as were used here are generally effective in coregistering structures close to control points, whereas structures further away are subject to greater error (
77). This is demonstrated in the present study by the correlation between coregistration accuracy and distance of landmarks from the centroid of the brain. Thus, while the Talairach method is widely accepted as a “precise quantitative framework for multimodality mapping.as well as coordinate-based morphometry” (
78), it has limitations in stereotaxic localization of cortical landmarks (
78,
79), with a residual error large enough to confuse adjacent gyri (
30). This is again consistent with the validity data from the present study demonstrating virtually negligible stereotaxic error near central structures, with a larger but still relatively small (less than 2 mm) error in cortical coregistration. In this regard, it should be noted that many of the regions in which significant signal intensity differences were found were in close proximity to either the anterior commissure or posterior commissure control points (e.g., periventricular differences) and may be expected to have high anatomic reliability.
Systematic misregistration is likely to produce signal intensity differences at brain/CSF interfaces because of the steep signal intensity gradient on this T1-weighted sequence, with brain/CSF contrast greater than 10:1. Signal intensity differences in this study were, indeed, limited mainly to brain/CSF boundaries. However, the multiple patchy areas of signal intensity differences at brain/CSF edges differ substantially from the more global, symmetric edge artifacts typically seen with misregistration. In addition, misregistration errors might be expected to result in signal intensity differences for analyses of both schizophrenic minus normal group values and normal minus schizophrenic group values. The absence of any significant differences in the analysis of schizophrenic minus normal group values argues against significant misregistration artifacts in the results reported here.
Last, it might also be argued that since overall sizing is based on the linear extent of brain in each dimension, the presence of cortical thinning in schizophrenic patients would result in a elongation of brain structures along that same dimension, since the images are being stretched to a larger degree than they would if the cortex were intact. (The same argument may be applied to all linear and planar ventricle-brain ratio measurements.) However, the magnitude of such thinning is trivial compared to the full linear extent of brain in any dimension (
80,
81). Since all slices for a given subject are resized (in each dimension) by a constant proportion for that subject, any such “overstretching” of central structures in cases with cortical thinning should then occur throughout the entire extent in the brain. For example, if images of schizophrenic subjects were overelongated in the left-right axis in the coronal plane, then a consistent signal intensity difference would be observed along the entire lateral and medial edges of the ventricular system from rostral to caudal extent. While there were, indeed, widespread periventricular signal intensity differences, these had a patchy, nonsymmetric pattern not suggestive of an artifact. In addition, as noted earlier, the finding of ventricular enlargement here was consistent with volumetric measurement of ventricular regions in these patients (M. Sanfilipo et al., unpublished data).
In sum, these considerations lend credence to the validity of the proportionate averaging method as an exploratory tool for the description of structural differences across populations. The comparative ease, speed, and reduction in labor intensity make it particularly attractive for analysis of large samples. Such a global analysis is of particular value in conveying a broader gestalt of morphologic deviation, as demonstrated here by the visualization of diffuse differences in T1 signal intensity in schizophrenic patients.
APPENDIX 1. Image Averaging Methods
1. Reangulation
In the description below, the spatial coordinate x is defined as the left-right axis, y as the superior-inferior axis, and z as the rostrocaudal axis. The initial step entails correction for misangulation of an individual brain from the ideal orientation based on the Talairach (
37,
38) coordinate system. In this system, the plane of interhemispheric fissure is taken as the yz plane, and the line the connecting anterior and the posterior commissures defines the direction of the y axis.
First, with the mouse-driven cursor several points are located on the interhemispheric fissure on five coronal images spaced by 2 cm. Equations are then determined for straight lines that approximate the average orientation of the interhemispheric fissure. Each scan is rotated about the y axis by the angle equal and opposite to the average deviation of these lines from the ideal vertical orientation.
Next, a similar process is applied to the interhemispheric fissure, as seen in several axial reformats of the scan. The second rotation of the scans, this time about the z axis, realigns the scan so that the interhemispheric fissure is parallel to the yz plane. The misangulation measured and corrected in these two steps never exceeded a maximum absolute deviation of 2.2 degrees, and it averaged an absolute deviation of 1.1 degrees.
The final reangulation consists of the measurement of the slope of the line supporting the ventral aspects of the anterior and posterior commissures seen in the midsagittal plane. In this data set, this line was oriented, on average, 19.1 degrees with respect to the original xy plane. In addition, the variability of this angle was several times larger than the misangulation of the interhemispheric fissure. The absolute deviation from the average had a maximum of 9.5 degrees and an average of 3.8 degrees. After performance of this angular measurement, each scan is realigned to the orientation of the anterior commissure-posterior commissure line equal to the average. (The reangulation routines, as well as the stretching routines, described below require that the image be interpolated, which was performed by using a trilinear interpolation method.)
2. Linear stretching
The next series of measurements are used to ascertain the maximum extent of the brain parenchyma, which is needed to spatially normalize the images. The image analysis software employed for this project allows the user to view axial (xy), coronal (xz), and sagittal (yz) sections. Moreover, successive sections can be scrolled and the voxel coordinates of specified points recorded. Through use of these interactive tools, extreme points on the brain surface may be readily identified. All measured values were integers expressed in pixels (0.96 mm). The detailed procedure was as follows.
a. Five coordinates are recorded on the axial views: xamin, xa0, xamax, yamin, and yamax. Xamin is the minimum x coordinate, and xamax is the maximum x coordinate, with similar notations for the y coordinate. Note that the “min” and “max” are for entire brain volume and do not necessarily occur on the same slice. Xa0 is the coordinate of the midsagittal fissure as seen on the axial view.
b. Five coordinates are also recorded on the coronal views: xcmin, xc0, xcmax, zcmin, and zcmax. Xcmin is the minimum x coordinate, xcmax is the maximum x coordinate, and xc0 is the coordinate of the midsagittal fissure as seen on the coronal view. Zcmax is the uppermost voxel of the cortex. Zcmin was defined in this study as the base of the pons.
c. Finally, eight coordinates are recorded on the sagittal views: ysmin, ysmax, zsmin, zsmax, ysac, yspc, zsac, and zspc. Ysmin is the minimum y coordinate, ysmax is the maximum y coordinate, and zsmax is the maximum z coordinate of the cortex—all located within less than 5 mm from the midsagittal fissure. Coordinates ysac, yspc, zsac, and zspc are the locations of the anterior and posterior commissures on the midsagittal section. Zsmin is the base of the pons seen on midsagittal slice.
d. Validation and elimination of redundant measured data—theoretically, the extreme coordinates (e.g., xamin, xcmin) should be equal, since they measure the same extreme point (xmin) on two orthogonal views. In this step, redundant measurements are compared, and, if a discrepancy is found, the particular brain is remeasured, and the discrepancy is eliminated.
e. The validated coordinates are collapsed to nine values for each brain: xmin, xmax, ymin, ymax, zmin, and zmax—extents of the bounding box, and x0, y0, and z0. The last two coordinates are defined as y0=(yac+ypc)/2, z0=(zac+zpc)/2. The coordinates x0, y0, and z0 define the midpoint C of the segment connecting the anterior and posterior commissures. The collapsed values are averaged for the entire set of 50 scans.
f. Individual brains are now spatially normalized in terms of this average. In this translation step, all brains are translated so that point C (the midpoint of the anterior-posterior commissure segment line) is in the same location for all subjects.
g. Finally, the eight octants of the brain are linearly scaled to align their extreme points without disturbing the location of point C. Thus, on completion of these steps, all of the variance due to global cortical extent is eliminated in each of the six directions.
3. Signal intensity normalization
Average signal intensity values for white matter were determined for each subject by sampling multiple white matter regions of high anatomic confidence (e.g., centrum semiovale). A standardization value was calculated to normalize average white matter signal intensity values to a constant value for all subjects across all slices. This standardization value was then used to rescale on a pixel-wise basis signal intensity values from the scans of all subjects.