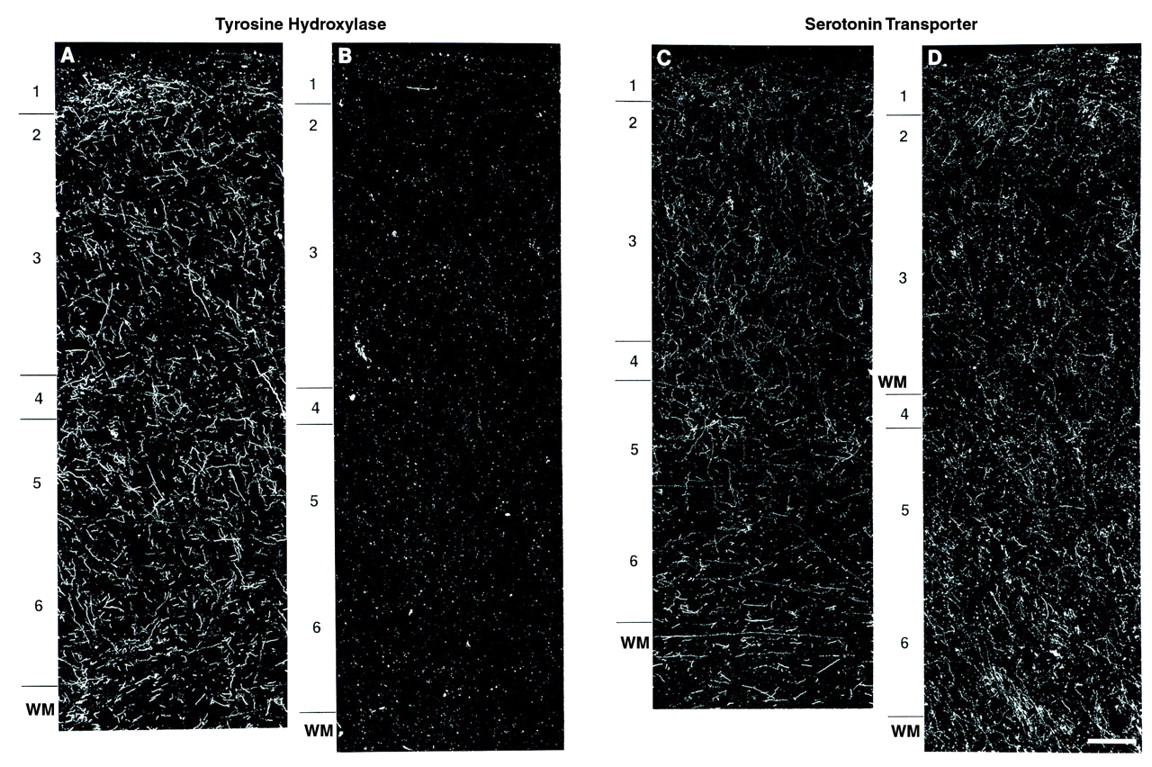
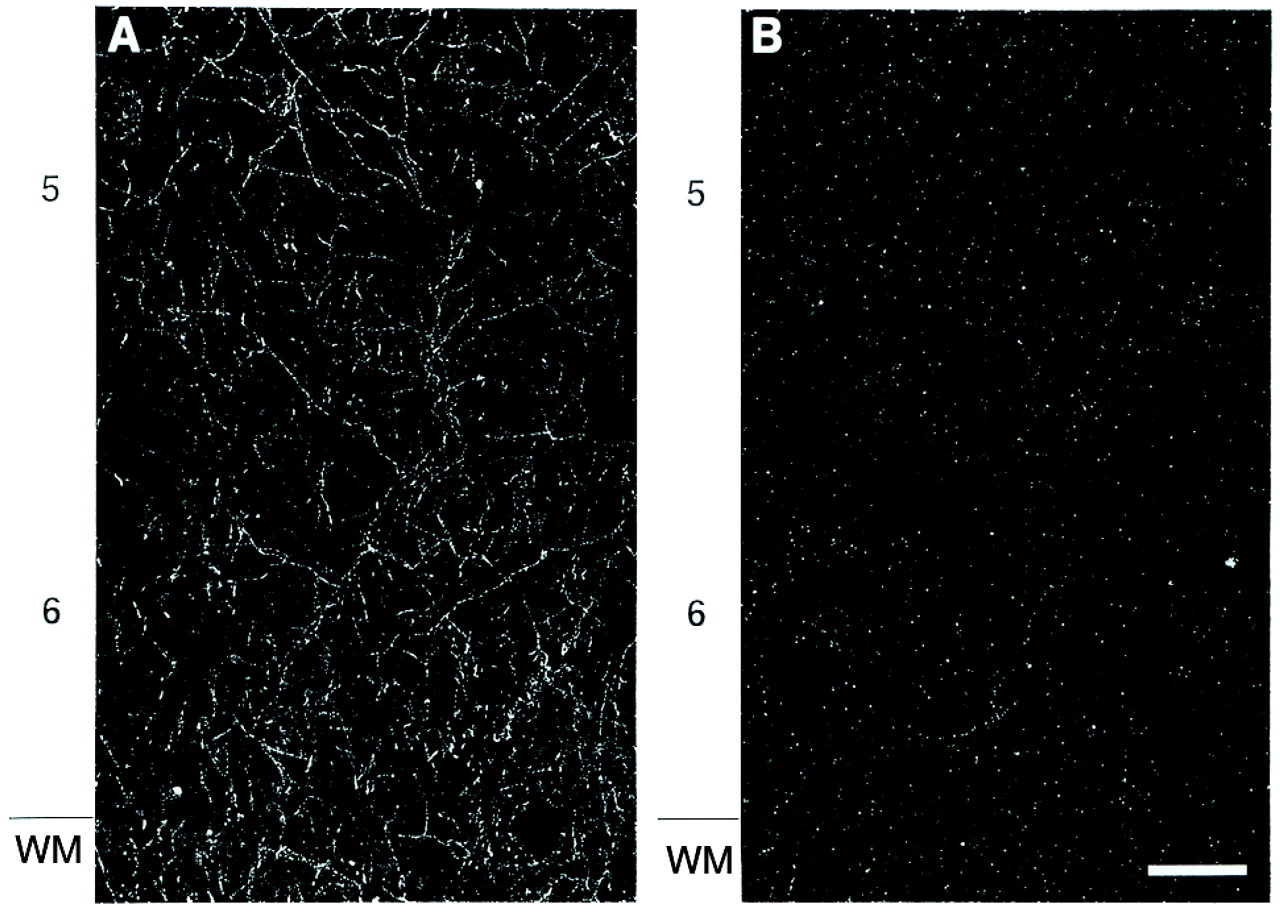
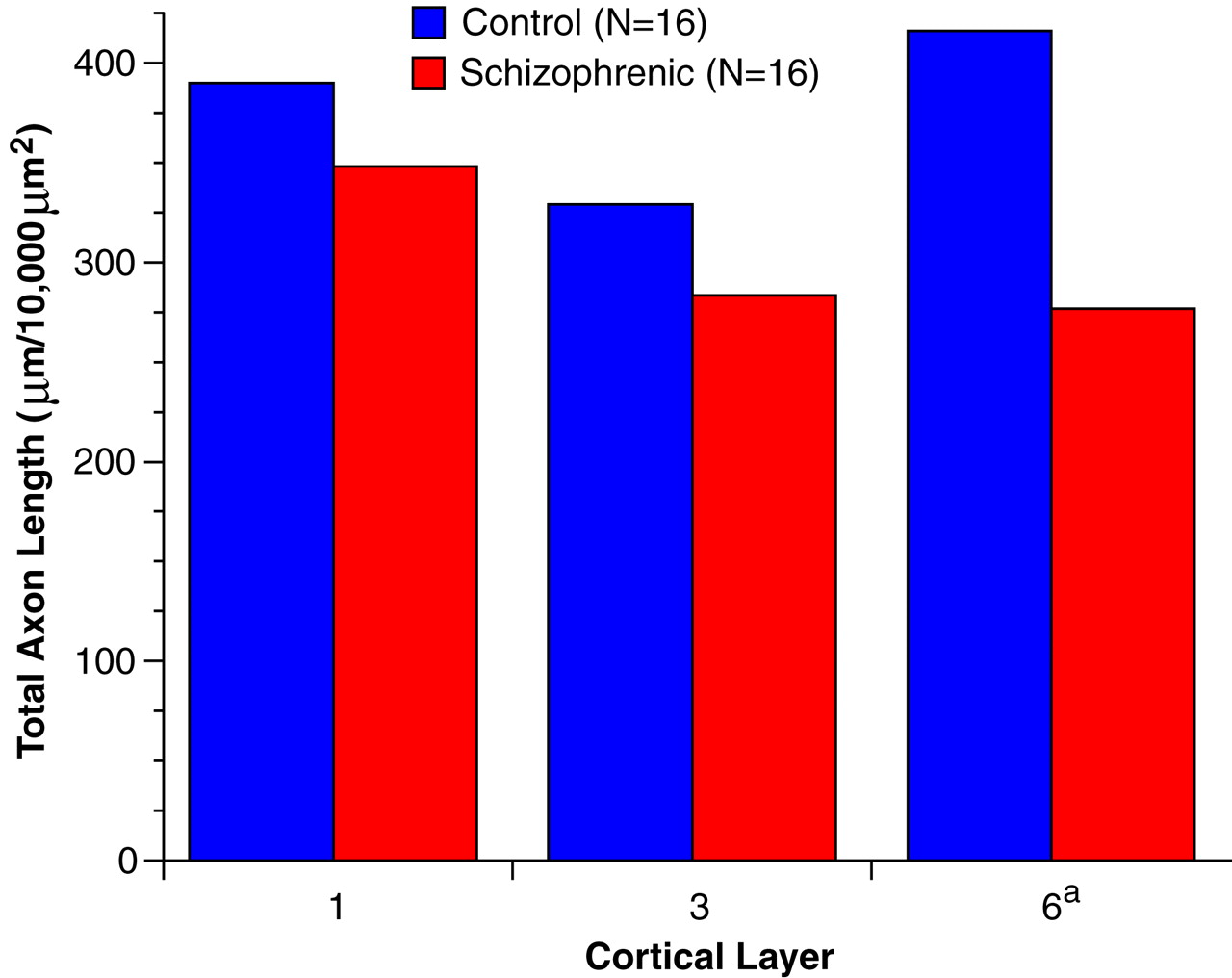
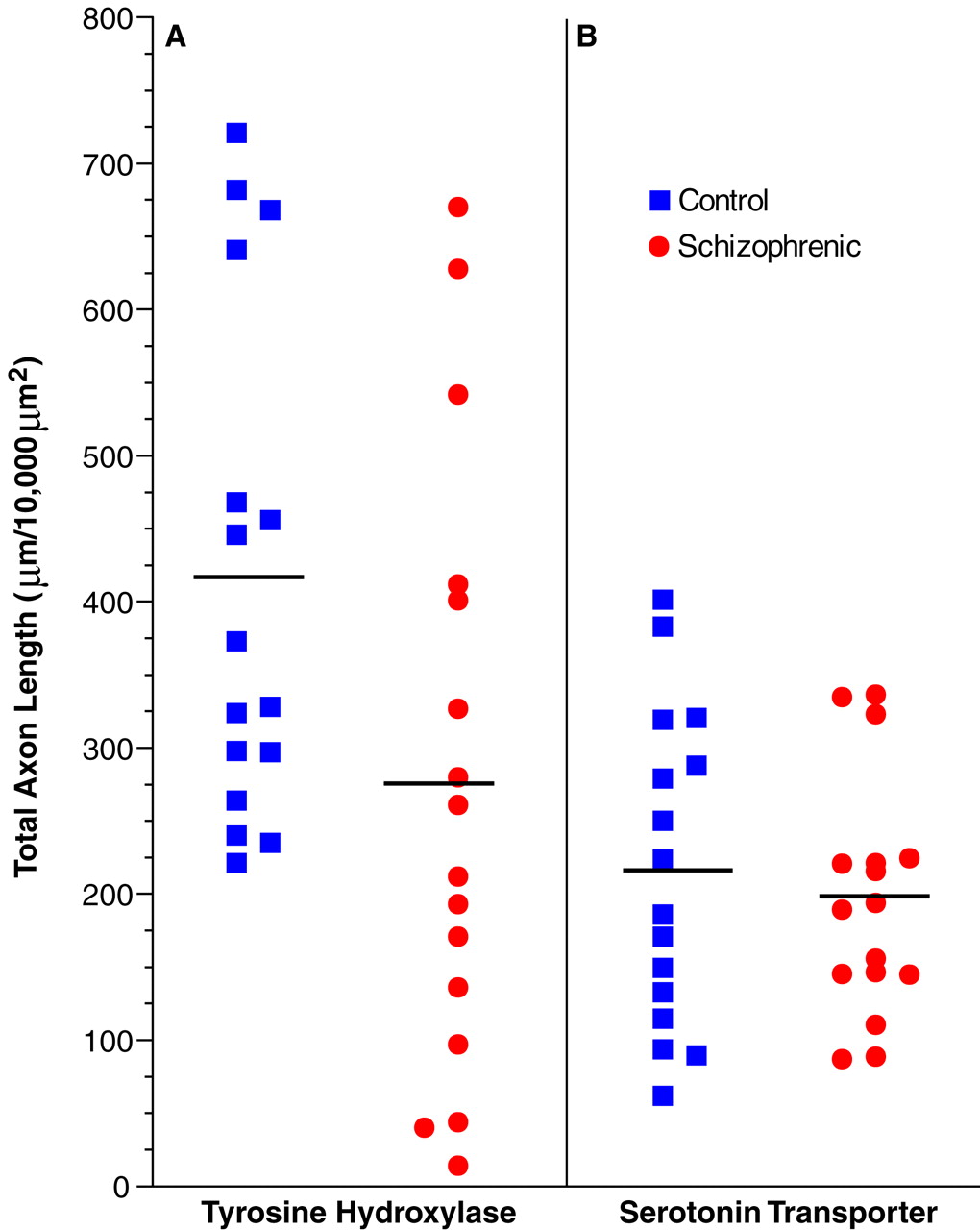
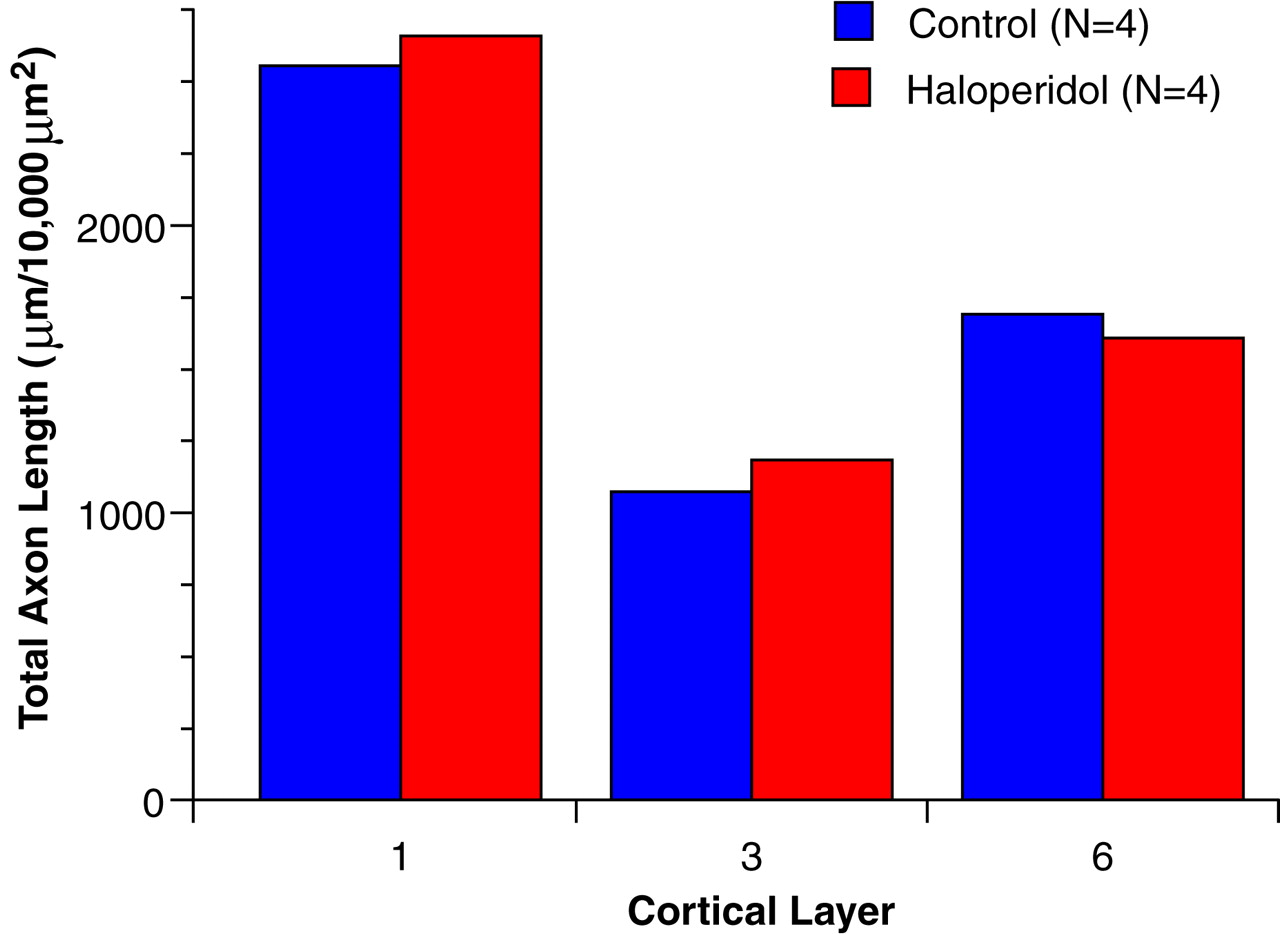
The results of this study demonstrate a significant reduction in the relative density of axons immunoreactive for tyrosine hydroxylase and dopamine membrane transporter, two proteins that play a critical role in dopamine neurotransmission, in layer 6 of prefrontal cortex area 9 in subjects with schizophrenia. In contrast, cortical afferents immunoreactive for the serotonin transporter, a central protein in serotonin neurotransmission, did not differ between schizophrenic and control subjects. Furthermore, chronic treatment with haloperidol and benztropine did not alter the density of tyrosine hydroxylase-immunoreactive axons in the monkey prefrontal cortex; this suggests that our findings are not likely to be a consequence of the typical treatments used in schizophrenic subjects. Together, these findings provide evidence that the pathophysiology of schizophrenia includes a lamina- and neurotransmitter-specific alteration in the dopamine innervation of the prefrontal cortex.
Specificity of Alterations to Dopamine Innervation of the Prefrontal Cortex in Schizophrenia
The dopamine membrane transporter and tyrosine hydroxylase antibodies used in this study have both been shown to specifically label dopamine axons in the primate neocortex. The mRNA for the dopamine membrane transporter is expressed selectively in dopamine neurons, and the protein is confined to the cell bodies, dendrites, and axonal projections of these neurons
(25). Although tyrosine hydroxylase is expressed in all catecholamine-containing neurons, multiple lines of evidence indicate that antibodies against tyrosine hydroxylase predominantly label dopamine axons in the primate neocortex
(22). In both monkeys and humans, the distribution and morphology of cortical tyrosine hydroxylase-immunoreactive axons differ from those of axons immunolabeled for dopamine-beta-hydroxylase, a marker of noradrenergic structures
(18,
35), and few cortical axons exhibit both tyrosine hydroxylase and dopamine-beta-hydroxylase immunoreactivity
(36,
37). In contrast, direct comparisons of tyrosine hydroxylase-immunoreactive axons and those labeled with an anti-dopamine antibody revealed identical patterns of distribution in the monkey cortex
(17,
37), and over 95% of all tyrosine hydroxylase-immunoreactive axons in the monkey prefrontal cortex are also dopamine membrane transporter-immunoreactive
(19).
While our findings of a reduced density of tyrosine hydroxylase- and dopamine membrane transporter-immunoreactive axons are indicative of an alteration in dopamine afferents, they do not preclude the possibility of a concomitant reduction in other afferent systems to the prefrontal cortex in schizophrenia. Indeed, the magnitude of the reported decrease in synaptophysin
(20,
38), a marker of axon terminals, in the prefrontal cortex of schizophrenic subjects cannot be accounted for solely by reductions in dopamine afferents. However, the absence of a difference between schizophrenic and control subjects in serotonin transporter-immunoreactive axons in the present study indicates that the observed change in dopamine axons does not reflect a nonspecific alteration in all monoaminergic projections to the prefrontal cortex in schizophrenia. These observations are also consistent with previous radioligand binding studies that found similar levels of serotonin uptake sites in the prefrontal cortex of schizophrenic and control subjects
(39,
40).
Specificity of Alterations to the Disease Process of Schizophrenia
Several lines of evidence suggest that the findings of this study directly reflect the pathophysiology of the disease process and do not represent an epiphenomenon of its treatment or other factors. The absence of a change in serotonin transporter-immunoreactive axons in concert with the laminar-specific reduction in dopamine axons argues against the possibility that the present findings are a consequence of differences in the quality of the tissue between the two groups of subjects. In addition, previous investigations that included many of the subjects in the present study failed to find differences between the schizophrenic and control subjects in other immunocytochemical markers
(41,
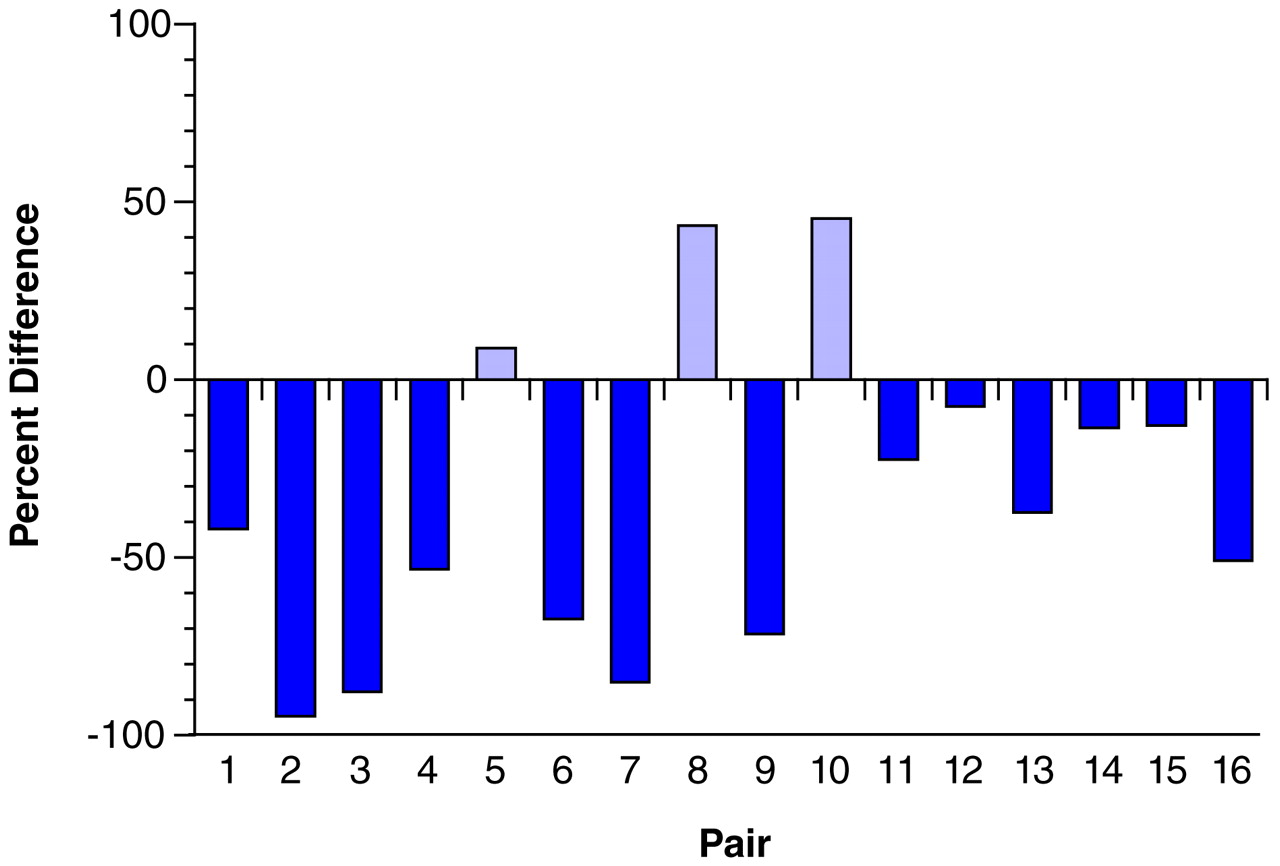
42). Most of the schizophrenic subjects included in the present study, but none of the control subjects, had been treated at some point in their lives with antipsychotic agents. However, the available evidence suggests that our findings are not the result of treatment with these agents, or of the anticholinergic drugs that are frequently used to treat the side effects of antipsychotic medications. First, our study included one schizophrenic subject who was never medicated and four subjects who were not receiving medications at the time of death. In each of these subjects, the density of tyrosine hydroxylase-immunoreactive axons in layer 6 was reduced compared to that in the matched control subjects (see pairs 1, 9, and 12–14 in
Figure 4). Second, chronic treatment of monkeys with haloperidol and benztropine did not alter the relative density of tyrosine hydroxylase-immunoreactive axons in the prefrontal cortex. The absence of an effect of antipsychotic medications on the measures used in this study is supported by a recent report that chronic haloperidol administration in the rat did not affect tyrosine hydroxylase immunoreactivity in neurons of the mesocortical dopamine system, although it did decrease tyrosine hydroxylase immunoreactivity in the nigrostriatal system
(43).
The possible influence of other clinical factors frequently associated with schizophrenia on tyrosine hydroxylase and dopamine membrane transporter immunoreactivity must also be considered. In the present study, six of the schizophrenic subjects had a history of alcohol abuse or dependence (
Table 1). However, nine of the 10 schizophrenic subjects without any history of alcohol abuse or dependence also had a reduced density of tyrosine hydroxylase-immunoreactive axons in layer 6 compared to the matched control subjects (compare
Table 1 and
Figure 4). In addition, in one of the pairs of subjects (pair 4 in
Table 1 and
Figure 4), both the schizophrenic and the control subject had a history of alcohol dependence, and yet the density of tyrosine hydroxylase-immunoreactive fibers was still decreased in the schizophrenic subject.
Pathophysiological Significance
The finding of a relative decrease in innervation density of both tyrosine hydroxylase- and dopamine membrane transporter-immunoreactive axons in the prefrontal cortex of schizophrenic subjects suggests that either 1) the concentrations of both proteins are reduced in a subpopulation of dopamine axons, such that these axons are no longer detectable by immunocytochemical techniques, or 2) the dopamine axons containing these proteins are decreased in number. Although the first possibility cannot be excluded, we are not aware of any data that would directly support this interpretation. On the other hand, a reduced number of cortical dopamine axons might be expected to be associated with alterations in the dopamine neurons of the ventral tegmental area, which furnish many of the dopamine afferents to the prefrontal cortex. In the only study that has examined dopamine neurons in schizophrenic subjects, the number of neuromelanin-containing cells in the medial mesencephalon was reported to be decreased by 16%, although this difference was not significant
(44). However, the somal volume of pigmented neurons was significantly reduced in the schizophrenic subjects. The latter findings could reflect a reduction in the size of the axon arbor of cortically projecting dopamine neurons, since somal size and total axon length tend to be positively correlated
(45,
46).
Either reduced amounts of tyrosine hydroxylase and dopamine membrane transporter in dopamine axons or the presence of fewer dopamine axons could be associated with diminished dopamine neurotransmission in the prefrontal cortex of schizophrenic subjects. This possibility is consistent with the interpretation of other studies (see introduction) suggesting that schizophrenia is associated with a hypodopaminergic state in the prefrontal cortex. In addition, animal studies suggest that even relatively modest reductions in the density of dopamine axons in the prefrontal cortex can be associated with a substantial decrease in indices of dopamine neurotransmission. For example, in contrast to the nigrostriatal dopamine system in which residual dopamine terminals can compensate for the loss of the majority of axons in this projection
(47), partial lesions of the dopamine mesocortical projection result in significantly decreased extracellular levels of dopamine in the prefrontal cortex
(33). We do not know whether the magnitude of the decrease in prefrontal cortex dopamine axons observed in the present study of schizophrenia is sufficient to produce the types of cognitive impairments previously associated with reductions of prefrontal cortex dopamine in nonhuman primates
(6–
8). However, the similarities in cognitive deficits observed in these animal studies and in subjects with schizophrenia
(48) suggest that the decrease in markers of prefrontal cortex dopamine axons observed in the present study could contribute to prefrontal cortex dysfunction in schizophrenia.
On the other hand, our findings of decreased tyrosine hydroxylase- and dopamine membrane transporter-immunoreactive axons do not necessarily provide evidence for a hypodopaminergic state in the prefrontal cortex of schizophrenic subjects. In fact, the complete absence of dopamine membrane transporter and reductions of 90% in tyrosine hydroxylase levels are associated with a markedly hyperdopaminergic state in the dopamine membrane transporter knockout mouse
(49). Some studies have indicated that prefrontal cortex function may be impaired by either a deficiency or an excess of stimulation at prefrontal cortex dopamine D
1 receptors
(7,
50–
52). Layer 6 of the primate prefrontal cortex contains a high density of dopamine D
1-like receptors
(53), and the density of these receptors has been reported to be decreased in drug-naive schizophrenic subjects
(11). Since the symptoms of schizophrenia are frequently worsened by stress, which increases prefrontal cortex dopamine release
(54), it is possible that decreases in pre- and postsynaptic dopamine markers in the prefrontal cortex represent a homeostatic response to minimize the impact of excessive levels of prefrontal cortex dopamine induced by environmental stress.
Although our findings reveal a lamina- and neurotransmitter-specific alteration in the dopamine innervation of the prefrontal cortex, other data suggest that these changes are likely to represent only one component of a more extensive set of alterations in prefrontal cortex circuitry in schizophrenia. For example, imaging and postmortem studies suggest that the mediodorsal thalamic nucleus, which furnishes the principal thalamic projection to the prefrontal cortex, may be reduced in size and contain fewer neurons in subjects with schizophrenia
(55,
56). In addition, findings from other studies support the notion that the thalamic projections to the middle layers of the prefrontal cortex are decreased in schizophrenia
(57,
58). Interestingly, the feedback projections from the prefrontal cortex to the mediodorsal thalamus originate from pyramidal neurons located in layers 5 and 6
(59), and the prefrontal cortex projections to the mediodorsal thalamus appear to play a prominent role in regulating thalamic activity (see reference
60 for a review). Since the dendritic shafts and spines of pyramidal cells are the principal synaptic target of dopamine axon terminals
(61), and dopamine appears to play a critical role in regulating the influence of other inputs on pyramidal cell activity
(62,
63), a shift in dopamine neurotransmission in prefrontal cortex layer 6 could reflect a change in the modulation of corticothalamic feedback in response to abnormal thalamocortical drive in schizophrenia.