RESULTS
Subjects’ ratings of the unpleasant (mean score=–6.12, SD=1.4), pleasant (mean=6.10, SD=1.1), and neutral (mean=2.38, SD=1.6) picture sets were consistent with the intended valence (F=358, df=2, 15, p<0.0001). Ratings of the neutral and pleasant stimuli were statistically different (F=57, df=1, 16, p<0.0001), as were the neutral/unpleasant (F=457, df=1, 16, p<0.0001) and the unpleasant/pleasant (F=680, df=1, 16, p<0.0001) comparisons.
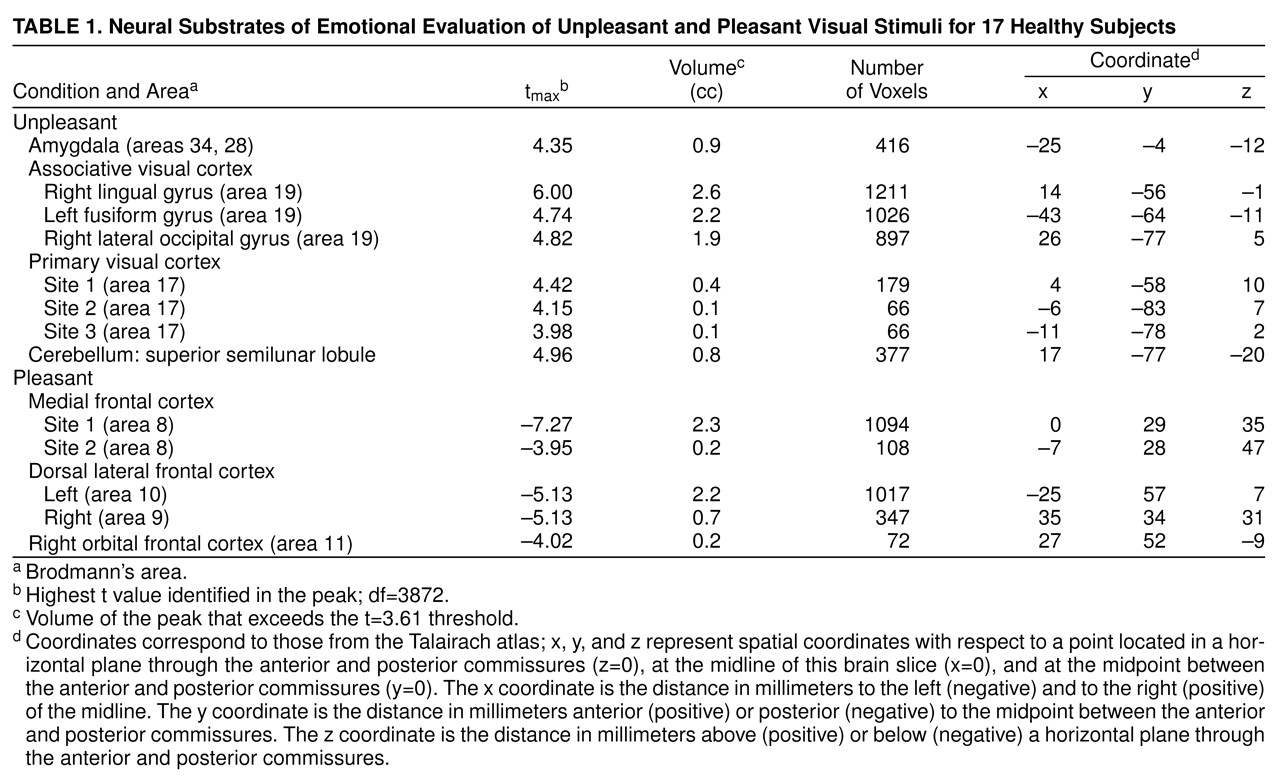
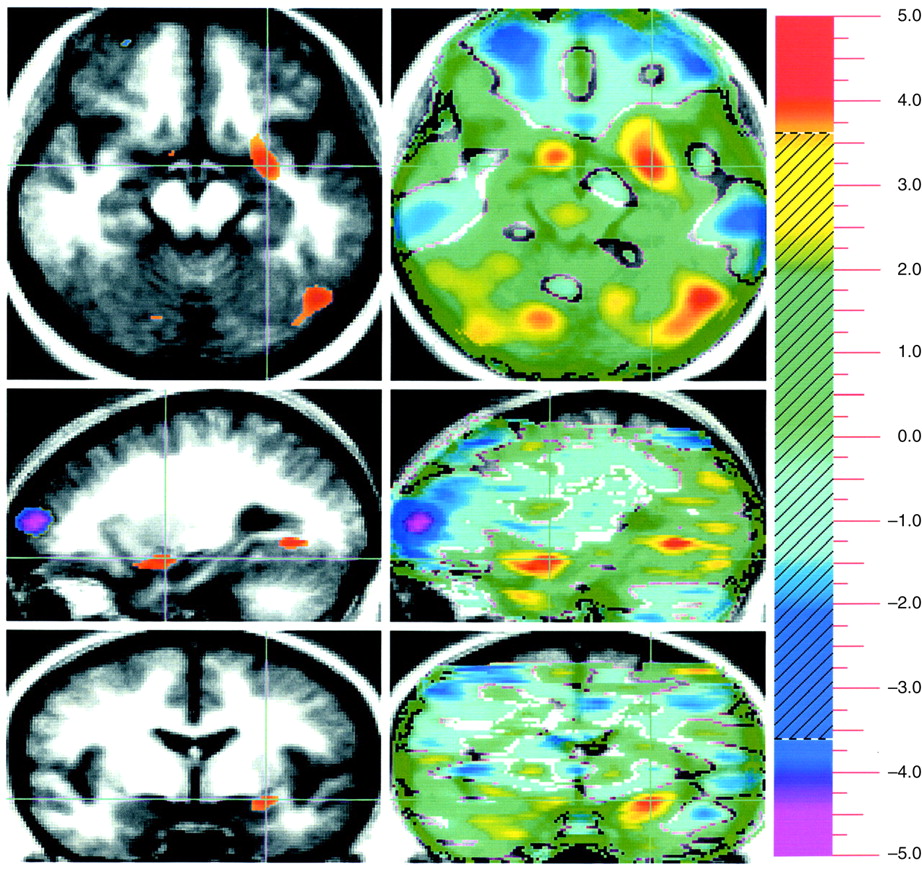
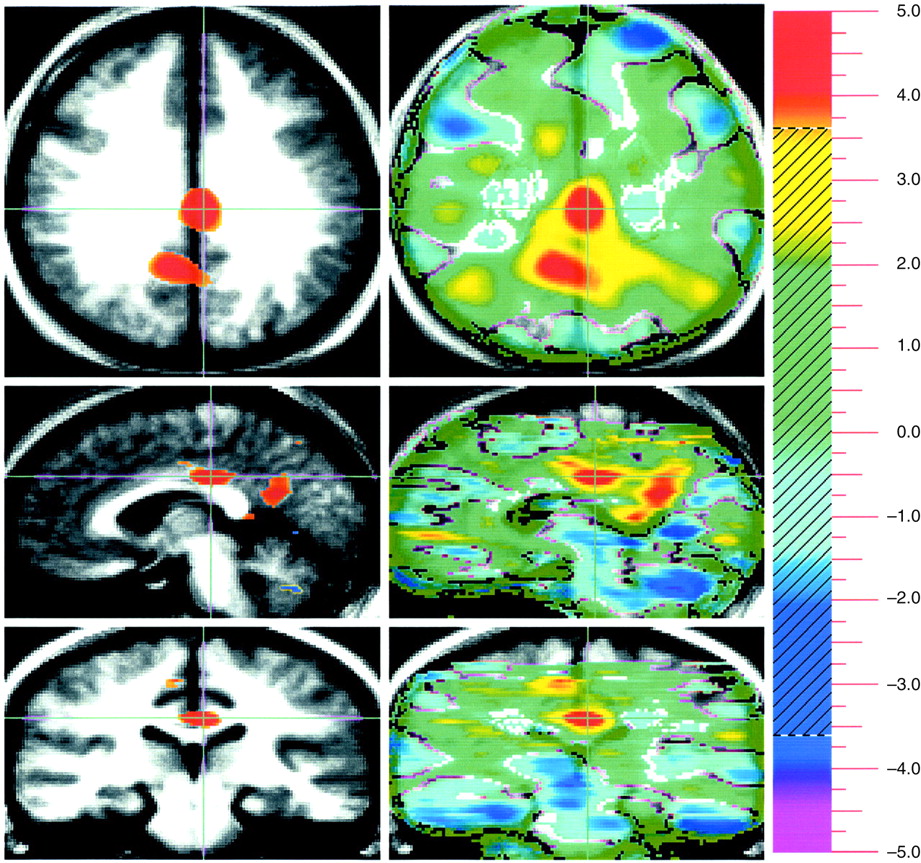
Evaluation of unpleasant visual stimuli relative to pleasant stimuli produced activations in primary and secondary visual cortex and in the superior semilunar lobule of the cerebellum. The left amygdala was relatively more active during evaluation of unpleasant stimuli (
Table 1 and
Figure 1).
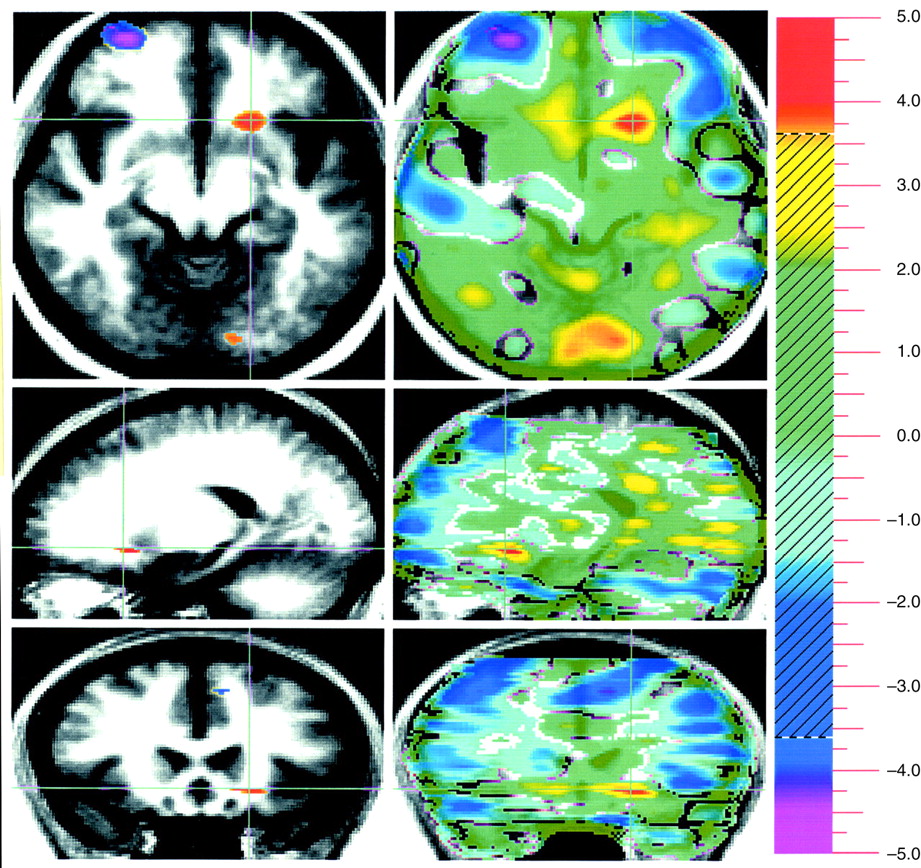
Evaluation of pleasant stimuli produced activations bilaterally in the medial, orbital, and dorsal lateral frontal cortex (
Table 1 and
Figure 1).
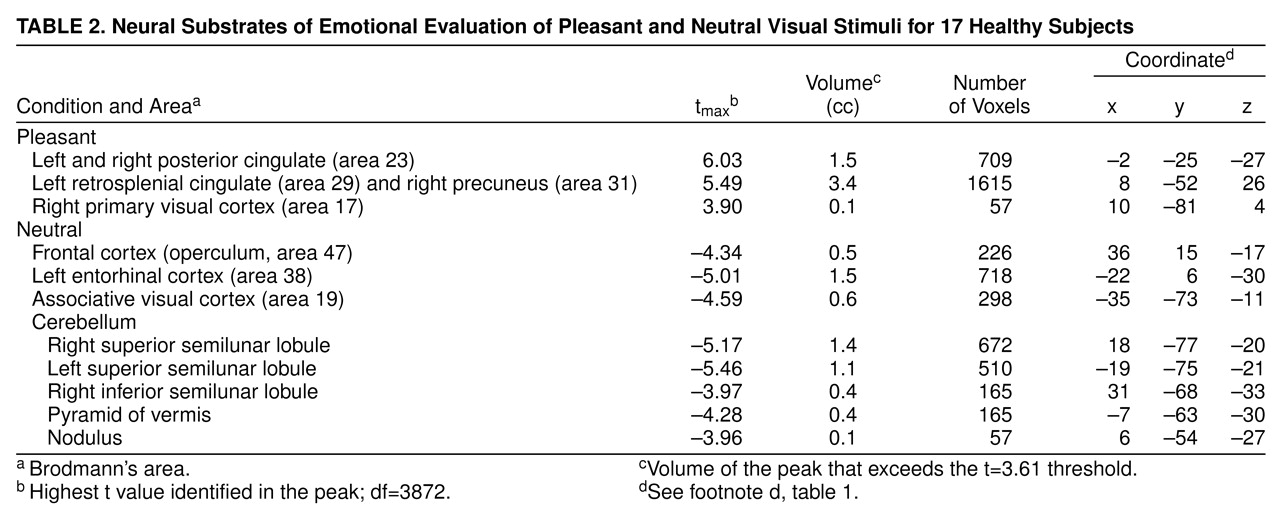
Subjects showed relatively higher blood flow in the primary visual cortex, left retrosplenial cingulate, and right precuneus in the pleasant picture condition. Bilaterally, the posterior cingulate gyrus was also more active (
Table 2 and
Figure 2) in the pleasant than in the neutral condition.
Evaluating neutral visual stimuli produced relatively higher blood flow, compared to the evaluation of pleasant pictures, in the visual association cortex, right frontal operculum, and entorhinal cortex. Several cerebellar locations, including the superior and inferior semilunar lobules, the pyramid of the vermis, and the nodulus, were also relatively more active (
Table 2 and
Figure 2).
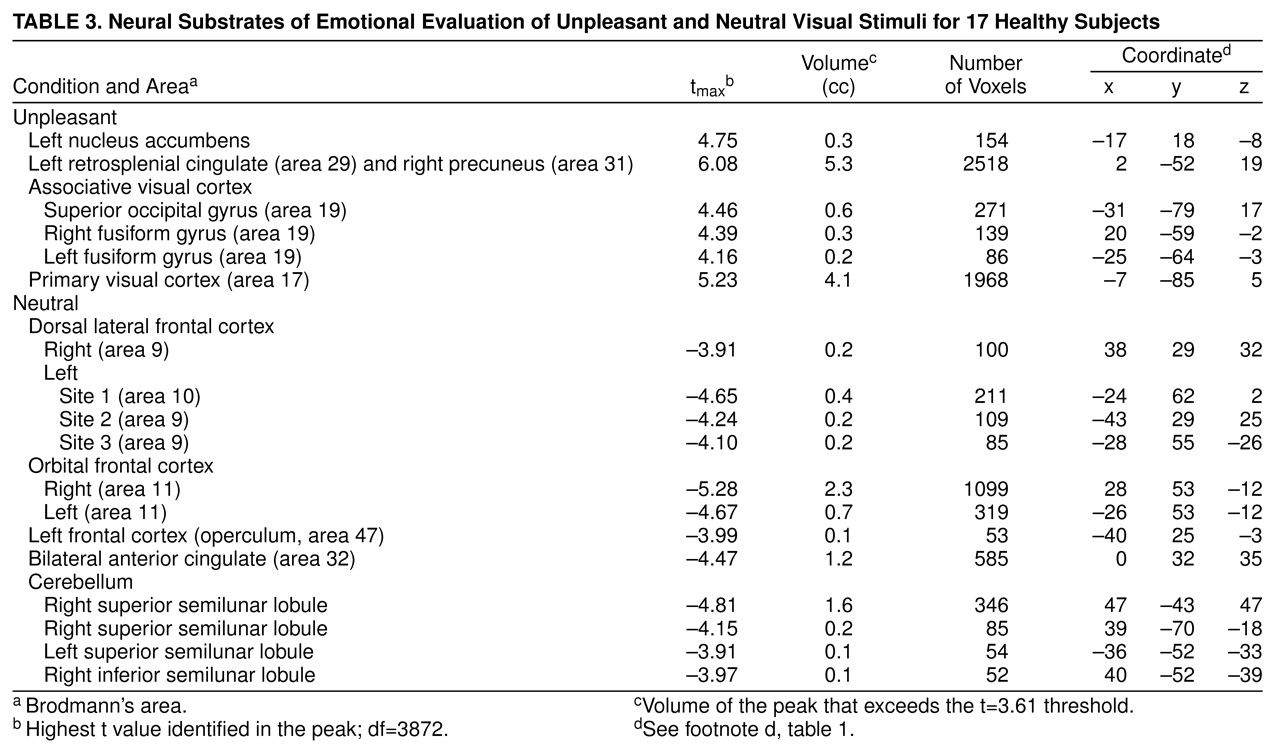
Evaluation of unpleasant stimuli produced activations in the primary and secondary visual cortex including bilateral fusiform gyri. The left retrosplenial cingulate gyrus and the right precuneus were relatively more active in a pattern very similar to that seen during evaluation of pleasant stimuli
Table 3 and
Figure 3). In addition, there was increased flow in the left nucleus accumbens. The neutral condition produced relative activations bilaterally in the dorsal lateral and orbital frontal cortex and in the left frontal operculum, bilaterally in the anterior cingulate, and in several locations in the cerebellum including the bilateral superior and right inferior semilunar lobules.
DISCUSSION
This study examined the functional neuroanatomy associated with the attribution of emotional valence to visual stimuli. Subcortical limbic regions were activated during attribution of valence to unpleasant stimuli, whereas cortical limbic areas were activated during attribution of valence to pleasant stimuli regardless of the comparison condition used. How may these findings be interpreted? Humans and nonhuman animals have very efficient neural mechanisms to detect danger that developed much earlier than the massive enlargement of the frontal cortex observed in primates. The danger recognition system, which is crucial for the survival of the species, evolved in order to function effectively with least cortical appraisal and thus is largely subcortical
(33). Human and nonhuman animals respond to a sudden danger in a somewhat stereotypic and universal way that initially does not involve complex cognitive processing and achieves promptness to escape. The ability to appreciate the positive aspect of events and situations, however, requires more sophisticated processing of the stimuli that is individually personalized and has the characteristic of a “higher” cortical process. It is thus arguable that the detection of pleasant features relies on a rather phylogenetically newer circuit that involves largely the prefrontal cortex and the cortical executive system.
A way of isolating a differential neural response to attribution of emotional valence in pleasant and unpleasant pictorial stimuli that is least influenced by arousal is to subtract these two conditions from one another. This subtraction showed increased activity in the amygdala associated with the attribution of emotional valence to the unpleasant stimuli, whereas emotional evaluation of pleasant stimuli was associated with several areas in the orbital medial and dorsal lateral prefrontal cortex.
The role of the amygdala in emotional behavior has been recognized
(34–
37). Studies using affective manipulations of sensory/cognitive tasks or drugs have associated the amygdala with emotions in humans
(4,
6,
8,
9,
14,
38–
41). Results from this study are consistent with research showing amygdala involvement in emotion evaluation, specifically in extraction of affective content from visual stimuli
(8,
9,
14,
42–
46).
Whether the amygdala may specifically process only one of several possible negative emotions
(9,
42) or play a very general role in all affective behavior
(47–
49) is one of the open questions in emotional neuroscience (14). In contrast to the view positing a role of the amygdala in the appraisal of only one negative emotion, e.g., fear
(42), the present study found that increased amygdala activity was associated with evaluation of a wide range of unpleasant stimuli grouped in a pictorial sequence.
Consistent with the majority of the literature
(8,
9,
42,
45), the present study found no evidence of human amygdala involvement in the attribution of positive valence to visual stimuli with pleasant content. Using picture sets derived from the same source
(50) and a similar study design, Lane et al.
(45) have shown left amygdala activation in response to unpleasant but not pleasant visual stimuli. The higher amygdala blood flow in response to unpleasant relative to pleasant stimuli, but not relative to neutral stimuli, is consistent with the observations of Morris et al.
(9). They reported left amygdala blood flow increases with increasing fearfulness and decreases with increasing happiness in the visual stimuli
(9). Rapid habituation to happy faces
(14) may also account for the absence of amygdala differential activity observed in response to visually presented pleasant stimuli. Furthermore, the human amygdala may be involved in the mental processes necessary to detect affective valence for both negative and positive stimuli in other cognitive domains
(47). In nonhumans, the amygdala participates in emotion processing for both negative and positive stimuli regardless of the delivery of the stimuli
(33,
48,
51).
Results from the present study do suggest, however, that in humans affective evaluation of pleasant stimuli in the visual domain is carried on with a substantial contribution from the frontal lobe. This is consistent with findings from a number of research areas that suggest that the orbital frontal cortex plays an important role in representing information about reinforcing stimuli. Specifically, lesions of the orbital frontal cortex in animals interfere with short-term memory of reward information. The animal no longer can discriminate between good and bad
(52). Neuronal cells in the orbital frontal cortex respond to information about reward or punishment
(53–
55).
The present study is also consistent with previous research in healthy humans and individuals with clinical depression. Human inferior, medial, and orbital prefrontal cortices play an important role in emotional cognitive processes
(3,
4,
40,
56) including recognition of facial emotion
(57). Frontal regions are also engaged in the assessment of facial attractiveness
(58). Patients with full-blown depression, a condition that affects the patient’s ability to detect and take pleasure in joyful events and situations, show decreased perfusion in the left dorsal lateral and bilateral medial prefrontal regions
(59,
60). In patients with brain injury, damage to the left dorsal lateral frontal cortex is associated with clinical depression
(61–
65). Damage to the orbital frontal cortex is also associated with diminished ability in social decision making and detection of emotional clues
(66,
67), leading to changes in personality
(68). Taken together, these findings support the notion that the prefrontal cortex is part of a neural system engaged in detection of positive features in objects, events, and mental states.
The relative increased blood flow in the frontal lobe during the neutral/unpleasant comparison is consistent with the above hypothesis and may be explained by the different degree of pleasant valence detected by subjects.
Increased activity of primary and associative visual cortex was found in the unpleasant/pleasant comparison, as well as in the pleasant and unpleasant versus neutral comparisons. The observation of increased blood flow in the visual cortex during processing of emotional pictorial stimuli is frequent
(4,
7,
9,
45,
69) but poorly understood. Since the experimental and neutral stimuli in our and other studies
(4,
9) were matched for content complexity and luminance, it is arguable that some of the visual areas in the circuit may have been activated as a result of subcortical limbic back-signaling to visual cortex for a secondary assessment of the visual stimuli
(33). Consistent with previous studies
(45), visual association areas were relatively more active in the unpleasant/pleasant and unpleasant/neutral comparisons. Visual association areas may be preferentially involved in the evaluation of unpleasant visual stimuli in young individuals
(7,
46).
In the pleasant versus neutral subtraction, evaluation of pleasant pictures increased blood flow bilaterally in the posterior cingulate (Brodmann’s area 23). Functions attributed to the posterior cingulate include monitoring sensory events and the organism’s own behavior with respect to spatial orientation and memory
(70–
74). However, the role of the posterior cingulate as a component of the limbic system
(75) (and therefore in emotion) should probably be reevaluated in light of recent data. The posterior cingulate cortex of the macaque is strongly connected with orbital medial prefrontal cortex area 11m
(76) and participates in instrumental avoidance learning in the rabbit
(77). In humans, increased blood flow in the posterior cingulate is associated with classic conditioning
(78), comprehension of narratives necessitating the attribution of mental states
(79) or metaphors
(80), and recall of emotionally laden personal memories
(81). The left posterior cingulate showed increased activity during implicit recognition of emotion
(9). These and our findings suggest that the posterior cingulate gyrus may be involved in implicit and/or explicit evaluation of past and present contextual and emotional experiences.
Attribution of unpleasant valence relative to neutral showed relative increased activity in a nucleus of the basal forebrain: the nucleus accumbens. This subcortical nucleus, which is part of the limbic striatum
(82), receives major input from the amygdala
(83,
84) and may influence the anterior cingulate via the ventral pallidum
(85). Whereas the classic view has seen the nucleus accumbens playing a role in appetitive motivation and positive reinforcement
(86–
90), a variety of aversive and stressful situations (e.g., active avoidance behavior) increase dopamine release within the accumbens
(91–
95). Recently, the function of the accumbens has been conceptualized as linking motor and motivational processes that characterize goal-directed behavior
(96).
Relative to the neutral condition, evaluation of either pleasant or unpleasant stimuli activated the left retrosplenial cingulate (Brodmann’s area 29) and the right precuneus (Brodmann’s area 31). These shared neural substrates may represent memory component necessary during attribution of pleasant or unpleasant character to emotionally charged stimuli. Consistent with this hypothesis, the precuneus has been shown to be involved in episodic retrieval
(97) dependent on visual imagery
(98), as well as in recall, planning, and associative thinking
(99–
101). We posit that in order for humans to be consciously aware of a stimulus (and therefore examine its affective valence), the sensory representations of the stimulus need to be compared with past experiences of that stimulus and associated emotions
(33). This evaluation and its encoding in episodic memory, which involves the retrosplenial cingulate cortex
(97), are simultaneous processes.
Understanding the physiological meaning of areas of relative decreased blood flow is particularly challenging. Suspension of activity during the passive task or rest state
(102) or true neural inhibitions are proposed interpretations
(103). In the present study there was no “rest” state in that subjects attributed valence during all tasks. Therefore, neural inhibition or relative increased blood flow during the neutral condition
(7,
24,
99) may apply. These hypotheses help in the interpretation of two main findings in the present study that were not expected: the large cerebellar activation in the neutral condition (relative to both pleasant and unpleasant conditions) and the several frontal regions activated during neutral relative to unpleasant stimuli evaluation.
No verbal response or other movements were required during the PET experiment. Eye movement-related increased cerebellar flow during the most arousing tasks might have been expected in areas such as the vermis, fastigial nuclei, and floccular lobe
(104–
106). Instead, relative increased neocerebellar cortex blood flow was observed during the neutral condition. Consistent with a cerebellar role in cognition
(24,
107–
110) including attention
(111), this finding may be explained by a relatively more sustained attentional demand during the neutral condition in light of a decisional process evidently less straightforward compared to the pleasant or unpleasant condition. The increased blood flow in the anterior cingulate in the neutral/unpleasant comparison also points to a greater attentional load.
Whereas it is convenient under scholarly principles to divide emotion into evaluative, experiential, and expressive components, in real-life situations they may, albeit to a different degree, occur in some combination. Similarly to an ecological situation, the subjects in this study may have experienced “some” emotional arousal during attribution of valence. Experimental investigations using PET are currently under way in our laboratory to dissociate the functional neuroanatomy of evaluation and experience of emotion.
In conclusion, the present study has traced the functional neural anatomy associated with attribution of emotional valence in visual stimuli. Cortical limbic regions were associated with pleasant and subcortical regions with unpleasant valence regardless of the comparisons between the different experimental conditions. In the direct comparison, detection of pleasant stimuli displayed increased prefrontal activity, whereas detection of negative stimuli exhibited increased activity in the amygdala. Understanding the normal neural circuitry that supports the affective evaluation of events will help to uncover the neuroanatomical basis of psychiatric disorders with prominent emotional disturbances such as depression, anxiety disorders, posttraumatic stress disorder, and schizophrenia.