Clozapine and risperidone were the first two antipsychotic drugs of a new class of agents for the pharmacotherapy of schizophrenia. This class, which also includes olanzapine, sertindole, quetiapine, and ziprasidone, is distinguished from the typical neuroleptic agents by producing clinical efficacy with low levels of parkinsonian side effects or none. Although clozapine and risperidone were approved by the Food and Drug Administration in 1989 and 1993, respectively, and are now used worldwide, there are relatively few data from head-to-head clinical trials that have assessed their effects on symptoms, side effects, and neuroendocrine parameters in patients with schizophrenia. Thus, basic questions pertaining to their comparative efficacy remain unanswered.
It is reasonable to predict differences in clinical efficacy between clozapine and risperidone because they have some striking differences in their neurochemical properties and their effects in animal models. Even though both agents have a higher serotonin 5-HT
2A-to-dopamine D
2 binding ratio—a characteristic shared by the new-generation antipsychotics (
1)—clozapine has two orders of magnitude lower affinity for dopamine D
2 receptors and substantially lower affinity for 5-HT
2A receptors than risperidone (
2–
4). In addition, clozapine has severalfold greater antimuscarinic and antihistaminergic effects than risperidone (
2). In a primate model of acute dystonia, clozapine had essentially no associated dystonia, whereas risperidone exhibited dose-dependent effects (
5). Last, in clinically recommended doses, clozapine appears not to elevate plasma prolactin levels; the data on risperidone’s effects on prolactin are inconclusive (
6,
7). Lack of prolactin effects is a parameter proposed for defining the “atypical” profile of second-generation antipsychotic drugs (
8).
Given the interest in clozapine and risperidone, we decided to undertake a study of the comparative efficacy of these drugs for positive and negative symptoms, depression, parkinsonian symptoms, and neuroendocrine indexes in schizophrenic patients who met a priori criteria for partial response to traditional neuroleptic agents.
METHOD
Patients who participated in this study were enrolled in the Section on Clinical Studies treatment program at the National Institutes of Health Clinical Center, Bethesda, Md. Patients met the DSM-IV criteria for chronic schizophrenia as determined by an interview with the Structured Clinical Interview for DSM-III-R (
9) and a best-estimate diagnostic meeting. Information from the structured diagnostic interview was supplemented by data from past psychiatric records and from available informants. Patients with concurrent drug abuse, alcoholism, organic brain disorder, mental retardation, or a medical condition that contraindicates use of clozapine or risperidone were excluded from the study. The age range of the subjects was 18–55 years.
The patients met the following criteria for partial response to neuroleptics: 1) a history of residual positive and/or negative symptoms after at least a 6-week trial of a therapeutic dose of a neuroleptic agent, 2) at least a minimum level of positive and/or negative symptoms at the time of evaluation for the study, and 3) at least a minimum level of positive and/or negative symptoms after a prospective trial of at least 2 weeks of fluphenazine, 20 mg/day (with dose adjustments between 10 mg/day and 30 mg/day allowed in order to optimize outcome). The minimum positive symptom level was a total score of at least 8 for the four Brief Psychiatric Rating Scale (BPRS) (
10) positive symptom items (conceptual disorganization, hallucinations, unusual thought content, and suspiciousness). The minimum negative symptom level was a total score on the Scale for the Assessment of Negative Symptoms (SANS) (
11) of at least 20.
All patients were stabilized on a regimen of fluphenazine for a minimum of 2 weeks, with the exception of one patient who was maintained on thioridazine, 600 mg/day, because of a history of intolerance to fluphenazine. Then, a subgroup of the total study group underwent a drug-free period for participation in other research protocols. All patients were then randomly assigned to treatment with clozapine or risperidone in a 6-week, parallel-group, double-blind comparison trial. Over the first 2 weeks, study medications were gradually increased to 400 mg/day of clozapine and 6 mg/day of risperidone. Clinicians’-choice adjustments were permitted over the next 2 weeks within fixed limits (200 mg/day and 600 mg/day for clozapine and 2 mg/day and 9 mg/day for risperidone). Over the last 2-week period, study medications were held constant. Benztropine mesylate was allowed for extrapyramidal symptoms as needed, which was determined by blinded psychiatrists. Blood for monitoring WBC for agranulocytosis was drawn weekly from patients receiving both drugs to help maintain the blind.
Positive symptoms were assessed by the sum of scores on the four BPRS psychosis items indicated above. In addition, BPRS withdrawal/retardation and anxiety/depression factor scores and the total score on the 18-item BPRS (range=18–126) were used. The SANS total score (range=0–125) was used to assess negative symptoms.
The Simpson-Angus Rating Scale (
12) (range of scores=0–51) was used to assess parkinsonian symptoms. The item that rates salivation was omitted because increased salivation is a common side effect of clozapine that is not part of a parkinsonian clinical picture. The Hamilton Depression Rating Scale (
13) was used to assess depression. The symptom and parkinsonian ratings were conducted by a research psychiatrist blind to treatment assignment (two patients’ Hamilton depression ratings were unavailable for analysis). Monthly interrater reliability meetings were conducted throughout the study to minimize rater drift, and interrater reliabilities (intraclass coefficients) for the symptom scales were 0.75 or greater.
Venous blood samples were drawn between 7:00 a.m. and 8:00 a.m. from 13 of the 14 patients assigned to clozapine and 14 of the 15 patients assigned to risperidone during the last fluphenazine treatment week and week 6 of double-blind treatment. Samples were collected in EDTA-containing tubes, immediately placed on ice, and centrifuged for plasma separation; the resultant plasma was frozen until assay. Prolactin levels were assayed with microparticle enzyme immunoassay (intra-assay and interassay variability: 2.8% and 1.1%, respectively), growth hormone was assayed with radioimmunoassay (intra-assay and interassay variability: 9.2% and 6.6%, respectively), and cortisol was assayed with fluorescence polarization immunoassay (intra-assay and interassay variability: 3.0% and 5.2%, respectively).
In the statistical analysis, the efficacy of clozapine and the efficacy of risperidone were compared by using the data from the end of the double-blind study (week 6), with baseline ratings (i.e., those from the last week of fluphenazine treatment) as the covariates. The last week of fluphenazine treatment was used as the baseline because it provides the greatest generalizability of the findings to clinical practice (i.e., switching to atypical antipsychotics from chronic neuroleptic treatment) and maximizes uniformity across all subjects. There were no significant differences between clozapine and risperidone baseline data, and assumptions of parallel slopes were assessed before analyses of covariance. A secondary, within-treatment-group analysis was conducted by comparing fluphenazine baseline data with week 6 data for each study drug. Effect size was calculated for efficacy variables by dividing the treatment difference (clozapine change from baseline minus risperidone change from baseline) by the square root of the mean squared error.
RESULTS
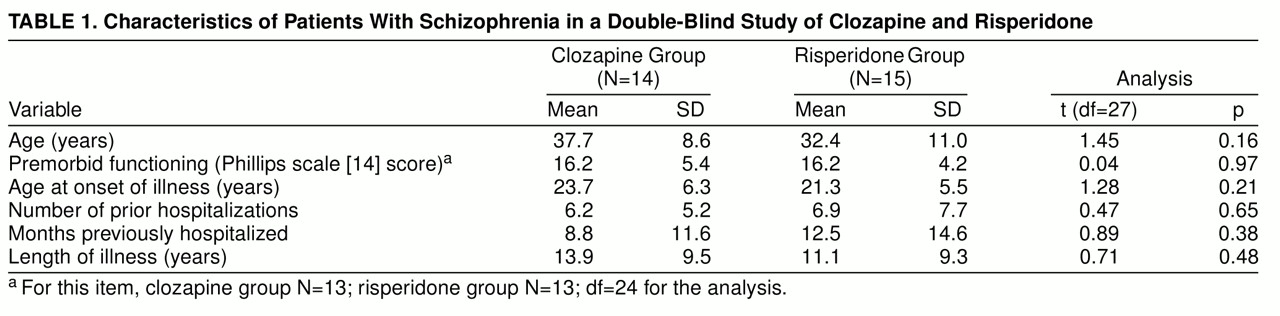
Twenty-nine patients entered the neuroleptic baseline phase and completed the 6-week double-blind comparative study; 14 (eight male and six female) were randomly assigned to clozapine and 15 (11 male and four female) to risperidone. There were no significant differences in the two groups’ demographic and clinical admission characteristics (
table 1). Nineteen of the 29 patients underwent a drug-free period before random assignment (nine patients subsequently assigned to clozapine had a mean of 19.4 drug-free days, SD=7.9, and 10 patients subsequently assigned to risperidone had a mean of 17.6 drug-free days, SD=8.2; t=0.50, df=17, p=0.60). Two prospective subjects relapsed before random treatment assignment, and three were not successfully changed over to fluphenazine for baseline assessments; these five patients were not among the 29 subjects in the study analyses.
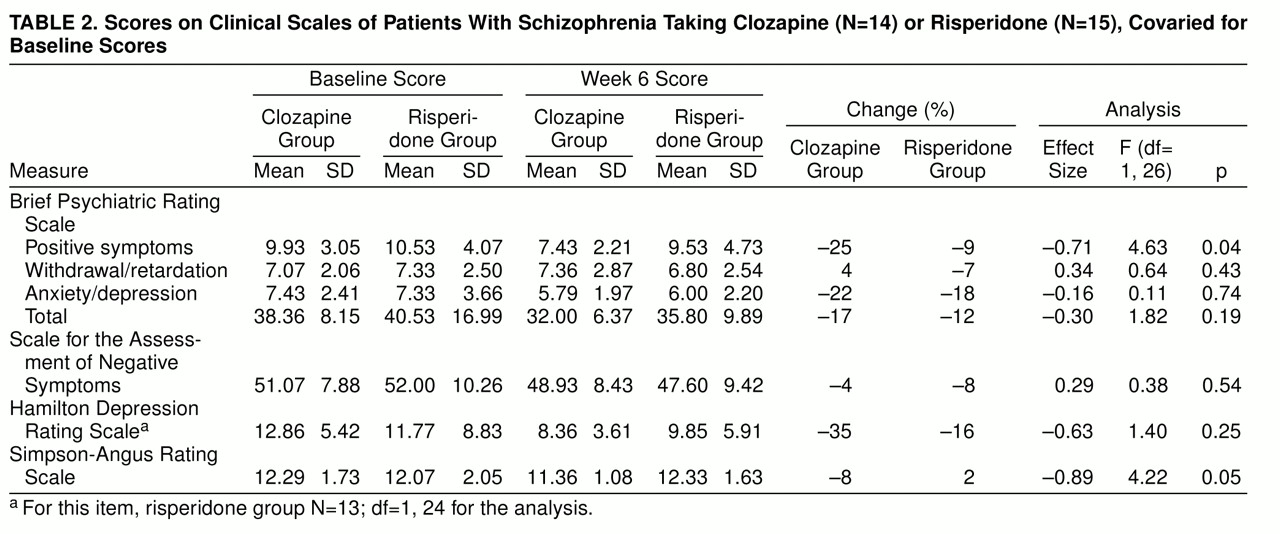
Clozapine was superior to risperidone for BPRS positive symptoms and Simpson-Angus scores, but study drug comparisons revealed no significant differences on two measures of negative symptoms (SANS score and BPRS withdrawal/retardation score), BPRS total score, BPRS anxiety/depression score, and Hamilton depression score (
table 2). A secondary within-group analysis comparing baseline and week 6 scores of the clozapine-treated patients revealed significant decreases in BPRS positive symptoms (F=13.9, df=1, 13, p=0.002), BPRS anxiety/depression (F=11.9, df=1, 13, p=0.004), BPRS total scores (F=18.9, df=1, 13, p=0.007), and Hamilton depression scores (F=13.0, df=1, 13, p=0.003). There were no significant differences between baseline and week 6 scores of the risperidone-treated patients. Using 20% change in BPRS total score to identify categorical responders, we found that five (35.7%) of the 14 patients assigned to clozapine and three (20.0%) of the 15 patients assigned to risperidone met the response criteria (χ
2=0.9, df=28, p=0.34).
The mean daily dose during week 6 was 403.6 mg/day (SD=79.6) for clozapine and 5.9 mg/day (SD=1.6) for risperidone. Three of the 15 risperidone patients had doses above 6 mg/day. Two patients assigned to clozapine and 10 patients assigned to risperidone received benztropine for extrapyramidal side effects during the trial (χ2=8.2, df=28, p=0.004).
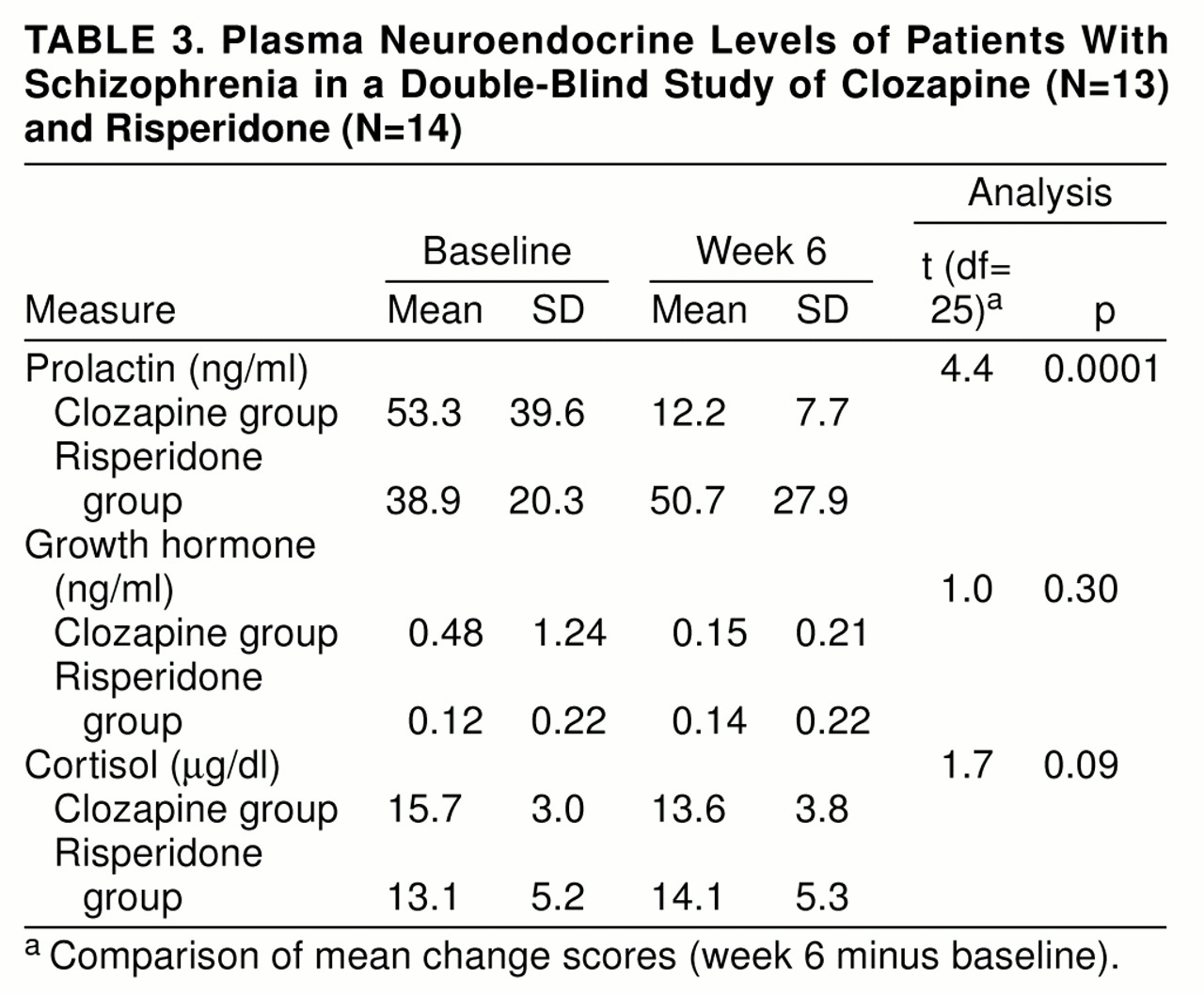
The assumptions of parallel slopes for the analysis of covariance for the prolactin data (F=8.9, df=1, 23, p=0.006) and the growth hormone data (F=4.7, df=1, 23, p=0.04) were not met, and therefore we did an analysis that compared the mean change scores (week 6 minus baseline) for the neuroendocrine data. There were significantly different effects of clozapine and risperidone on prolactin levels, with decrements from baseline to week 6 during clozapine treatment (
table 3). A secondary analysis including only male patients’ prolactin data was conducted, and the results were still significant (t=3.6, df=17, p=0.001). There were no significant differences between the drugs for cortisol and growth hormone (
table 3).
DISCUSSION
The results of this study indicate that clozapine is superior to risperidone for positive symptoms and extrapyramidal side effects, but differences were not found on two measures of negative symptoms, total symptom scores, and depression scores. Clozapine but not risperidone demonstrated significant reductions from fluphenazine baseline values in positive symptoms, total symptoms, and depression. In addition, clozapine produced fewer effects on plasma prolactin than either risperidone or fluphenazine, but significant differences were not found for growth hormone or cortisol.
The data demonstrating clozapine’s superior efficacy for psychosis are consistent with data from previous clinical trials. In several studies (
15–
19), clozapine has shown superiority to traditional neuroleptics for positive symptoms. Although risperidone demonstrates superiority to placebo for positive symptoms, it has not consistently shown superiority to traditional neuroleptics for positive symptoms (
20–
23), suggesting that clozapine may be more effective for the psychosis of schizophrenia, particularly in more chronically ill, less neuroleptic-responsive populations. We found no significant differences between the two agents for negative symptoms as assessed with both the BPRS withdrawal/retardation subscale and the SANS total score. Moreover, neither drug demonstrated significant reductions in negative symptoms from the fluphenazine baseline. In several but not all previous studies (
15–
22,
24), both agents demonstrated superiority to neuroleptics for negative symptoms. However, in the one previous study of a priori defined partially responsive schizophrenic patients (
17), clozapine’s effects on negative symptoms were modest, suggesting that this subgroup may have relatively treatment-resistant negative symptoms beyond what is treatable with traditional neuroleptics (
17,
25). The baseline negative symptoms in the current study were determined after a fluphenazine trial. There were no significant differences in depression between the clozapine and risperidone groups in the comparative analyses; however, the clozapine-treated patients demonstrated reductions from fluphenazine baseline in depression scores, which is consistent with previous reports noting clozapine’s greater efficacy for mood symptoms in comparison with neuroleptic drugs (
15,
26).
In two comparative clinical trials (
27,
28), clozapine and risperidone were found to have similar effects on symptoms. Klieser et al. (
27) included patients with acute exacerbation of symptoms who did not meet a priori criteria for lack of response to treatment or partial response. Thus, differences in patients’ clinical status at the time of study entrance (acutely ill versus stabilized on fluphenazine) and patient subtype (less chronically ill versus partially unresponsive to neuroleptics) may account for the differing results in the Klieser et al. study and the present study. Other differences that may have contributed to the discrepant results were a shorter treatment period (28 days) and fixed dosing arms (risperidone, 4 mg/day and 8 mg/day, and clozapine, 400 mg/day) in the study by Klieser and colleagues as opposed to flexible dosing designed to optimize clinical outcomes in the current study. In the study by Bondolfi et al. (
28), the mean daily dose of clozapine (291.2 mg) was lower than the dose in the present study and in other North American trials in treatment-resistant or partially responsive patients, which may account for differences between the results of their study and ours. In addition, the number of months of previous hospitalization in the study by Bondolfi and colleagues was approximately twice that in the current study, suggesting that their subjects may have been more treatment-resistant and chronically ill than our subjects. The Bondolfi et al. study’s treatment duration (8 weeks), risperidone dose (6.4 mg/day), and sociodemographic composition of the sample were roughly similar to those in the current study and are not a likely explanation for the discrepant findings.
The lower incidence of extrapyramidal side effects associated with clozapine is consistent with previous data from primate models and clinical studies. Casey (
5) demonstrated that risperidone has a steep dose-response curve for inducing dystonia in nonhuman primates—a predictive model of liability to extrapyramidal side effects in clinical populations. In contrast, even with exceedingly high doses, clozapine does not produce dystonia in primate models(
5). In clinical populations, risperidone is associated with dose-dependent induction of parkinsonian symptoms, with extrapyramidal side effects occurring in the upper dosage range (>6 mg/day) (
20,
22). In contrast, clozapine is rarely associated with induction of extrapyramidal side effects in clinical populations, even at high dosages (
15–
18). Our finding of a lower use of benztropine in clozapine-assigned patients than in those receiving risperidone supports the more favorable extrapyramidal side effects profile of clozapine.
Clozapine’s relative lack of effects on plasma prolactin levels compared with risperidone and fluphenazine is consistent with previous reports (
6,
7,
29) and is probably attributable to those agents’ differential effects on dopamine neurotransmission. Dopaminergic tuberoinfundibular pathways have an inhibitory effect on pituitary prolactin release that is disrupted with administration of potent dopaminergic antagonists. Both in vitro binding studies and in vivo human imaging studies demonstrate higher dopamine D
2 binding for neuroleptics and risperidone in comparison with clozapine (
2–
4,
16,
30,
31, and unpublished manuscript by Su et al., 1996). Moreover, in normal cultured rat pituitary cells, clozapine does not interfere with dopamine-mediated prolactin inhibition (
6). Since antipsychotic-drug-related extrapyramidal side effects and prolactin elevations tend to be associated, it is tempting to speculate that lower doses of risperidone that do not induce extrapyramidal side effects would be less likely to induce prolactin increases.
A few caveats should be considered in interpreting the findings of this study. The optimal dose of risperidone for comparative clinical trials is unclear. According to the literature (
20,
22), lower doses (i.e., <6 mg/day) are associated with a more favorable extrapyramidal side effects profile and perhaps better effects on negative symptoms, and prescribing trends demonstrate greater utilization of lower risperidone doses (e.g., 4 mg/day). Even though only 20% of the risperidone-assigned patients in the current study had doses in excess of 6 mg/day, it will be important to examine the efficacy of lower risperidone doses in this population. The trial duration (6 weeks) and study group size (N=29) may have limited the detection of differences in specific domains, such as negative symptoms and rates of response. The patients participated in a prospective trial of fluphenazine to establish partial response to neuroleptics and to allow baseline ratings of psychopathology before random assignment to a drug. Thus, the patients’ baseline values reflected relative clinical stability, which may contribute to less variance and potential for symptom change than a baseline at the time of acute exacerbation. Given the a priori admission criteria, it is unclear whether our findings are generalizable to other schizophrenia subgroups.
In conclusion, the data presented here indicate that these two second-generation agents have both important differences and similarities in their efficacy in schizophrenic patients who are partially responsive to typical neuroleptics. Clozapine had advantages for positive symptoms, extrapyramidal side effects, and prolactin levels, whereas differences in negative symptoms, total symptoms, depression, growth hormone, and cortisol were not found. Future research in partially responsive patients that addresses methodological issues such as optimal dose and treatment duration are needed.