There has recently been considerable interest in the role of gender in the expression of schizophrenic disease manifestations. This interest stems from observed differences in the clinical presentation of men and women with this illness. It has been reported that women with schizophrenia have a better response to pharmacological treatment, better premorbid social functioning, better long-term outcomes, and a later age at onset than men
(1–
4). Also, men with schizophrenia may be more likely to present with prominent negative symptoms, whereas women with schizophrenia are reported to present with proportionally more positive symptoms
(5,
6). No compelling explanations have been provided for these variations in clinical presentation; however, they suggest that gender may be a significant factor in the pathophysiology of this illness.
Among normal individuals, gender differences have been observed in cognitive functioning, neuroanatomy, and brain metabolism. Men have been reported to perform better on tasks involving spatial abilities, mathematical reasoning, navigation, and target-directed motor skills. In contrast, women have been shown to have superior ability on tasks involving perceptual speed, verbal fluency, mathematical calculation, and recalling landmarks
(7). In addition to differences in total brain weight, normal male brains are 10% larger than normal female brains; neuroanatomical studies have also identified several dimorphic areas of the brain
(8). Witelson et al.
(9) reported an 11% greater cell density in the posterior superior temporal gyrus of female brains in Brodmann area 22. The volume of the bed nucleus of the stria terminalis was reported to be 147% larger in male brains than in female brains
(10), and the investigators could not attribute this finding to the greater brain size in men. Although the corpus callosum is smaller in female brains than in male brains, the posterior region has been reported to be larger in female brain specimens
(11,
12). The anterior commissure has also been reported to be larger in female brains as compared with male brains
(13). Functional imaging studies have shown significant metabolic differences between normal men and women. Gur et al.
(14) demonstrated greater resting metabolism in men than in women in temporal-limbic regions and the cerebellum and lower resting metabolism in the cingulate cortex. In contrast, Andreason et al.
(15) reported that women had greater resting glucose metabolic rates than men, and Baxter et al.
(16) reported a trend of higher glucose metabolic rates in women than in men. Shaywitz et al.
(17), using functional magnetic resonance imaging (MRI), reported that gender differences exist in the language areas of the brain, with men showing left inferior frontal gyrus activation during phonological tasks and women showing more diffuse and bilateral activation of the inferior frontal gyrus during such tasks. These examples of cognitive, structural, and functional dimorphisms suggest that there are fundamental differences in brain organization between men and women, which may have important implications for differences in clinical presentation and pathophysiology of psychiatric illnesses such as schizophrenia.
The possibility that differences in the clinical presentations of men and women with schizophrenia are related to morphological differences in particular structures of the brain is an important, ongoing area of investigation. We chose to focus on the temporal lobe on the basis of previous MRI and postmortem studies, which have consistently implicated altered structure of the temporal lobe and its components (superior temporal gyrus and amygdala/hippocampal complex) in the pathophysiology of schizophrenia
(18–
36).
Several previous MRI studies have examined the issue of gender differences in temporal lobe structures of patients with schizophrenia. Bogerts et al.
(18) reported significant differences in the left posterior hippocampus, the right and left amygdala/hippocampus complex, the left anterior temporal horn of the ventricular system, and the right temporal lobe in male patients with schizophrenia as compared with male control subjects. There were no such findings in female patients and control subjects. Cowell et al.
(19) reported decrements in the left temporal lobe total volume in men, but not in women, with schizophrenia. Schlaepfer et al.
(20) examined 32 male and 14 female patients with schizophrenia and found reductions in gray matter in the superior temporal and middle temporal regions; these findings were most marked in the female patients. However, these authors did not state whether male/female differences were significant. In contrast, Flaum et al.
(21) examined 70 male and 32 female patients with schizophrenia and 45 male and 42 female comparison subjects, assessing several structures including the temporal lobe. They found that the patients with schizophrenia had smaller hippocampal and superior temporal gyrus volumes than the comparison subjects; however, no gender differences were found in this study. One major reason for differences in the results of the studies mentioned above may be differences in MRI acquisition parameters and slice thickness.
In the present study, volumes of the superior temporal gyrus, the amygdala/hippocampal complex, and the temporal lobe (excluding volumes of the superior temporal gyrus and amygdala/hippocampal complex) were assessed by MRI for gender differences in morphology in patients with schizophrenia and comparison subjects. Prefrontal cortex total volume, gray and white matter volumes, and caudate volume were also measured to test the specificity of temporal lobe results.
METHOD
Fifty-nine right-handed patients (23 women and 36 men) were selected from the Maryland Psychiatric Research Center outpatient research program (
table 1). The patients met the DSM-III-R criteria for chronic schizophrenia. A best-estimate diagnostic approach was used that incorporated all available sources of information, including direct assessment, family informants, and past medical and psychiatric records. Patients who had a DSM-III-R diagnosis of alcohol or substance abuse or dependence, organic brain disorder, or mental retardation or had a medical condition that may affect brain function and structure were excluded from the study. All patients were clinically stable on a regimen of antipsychotic medication. Thirty-seven right-handed comparison subjects (18 women and 19 men) were selected from the general population (
table 1). All comparison subjects were interviewed with the Structured Clinical Interview for DSM-III-R—Patient Version
(37). Potential comparison subjects with a history of a DSM-III-R axis I or axis II disorder, a family history of psychotic illness, or any medical condition known to affect brain function or structure were excluded from the study.
Handedness for all subjects was determined with the use of the Annett Handedness Scale
(38). All subjects in the study were between the ages of 21 and 45 years. Seventeen male comparison subjects were white and two were black; 13 female comparison subjects were white, three were black, and two were Asian. Twenty-seven male patients were white and nine were black; 14 female patients were white, eight were black, and one was Asian. After a complete description of the proposed study was discussed with each potential participant, written informed consent was obtained. Some subjects were part of an earlier MRI study that included 44 patients with schizophrenia (29 male and 15 female) and 29 comparison subjects (20 male and nine female)
(25).
MRI scans were obtained on a 2-T Siemens scanner operating at 1.5 T at the University of Maryland School of Medicine, Baltimore. A sagittal scout series was acquired first to correct for obvious head tilt and to localize imaging coordinates. Next, the whole brain was scanned with spin echo T1-weighted images. These T1-weighted images were obtained in the coronal plane with 3-mm contiguous sections. TR was 600 msec and TE was 17 msec, with two excitations and a 256×256-pixel matrix. MRI data were directly transferred from magnetic tape to optical disks for archiving. All identifying information was removed from the images for blinded assessment of morphology.
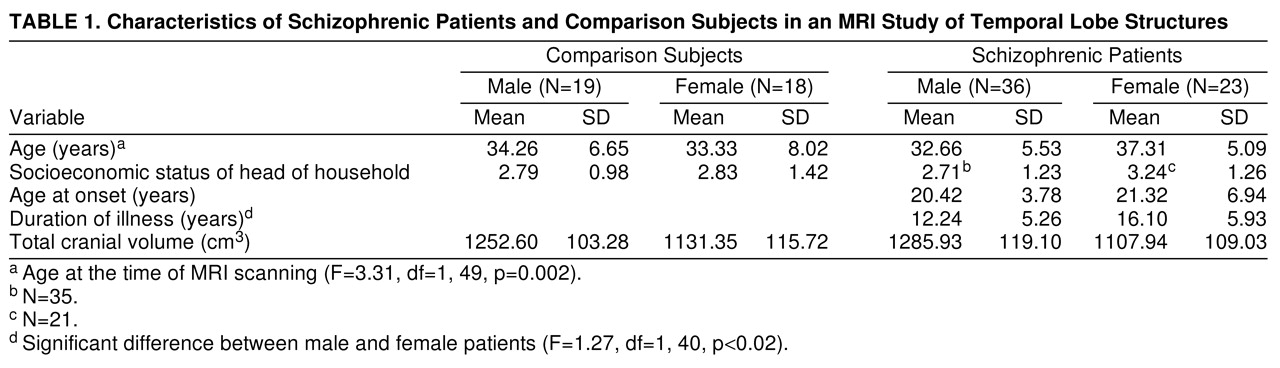
A system of rules based on brain atlases and published MRI studies was used to establish landmarks for delineating the boundaries of the regions of interest
(39–
43). These landmarks served as general guidelines to supplement the information derived from visual inspection of the MRI images. Landmark descriptions, measurement rules, and interrater reliability statistics for the prefrontal cortex total volume, the volumes of the prefrontal cortex white matter and gray matter, caudate, and amygdala/hippocampal complex, and total cranial volume have been previously established and reported
(25,
43). Landmark descriptions and measurement rules for the temporal lobe and the superior temporal gyrus were established in the same manner for the current study (
figure 1). The anterior boundary of the temporal lobe began at the temporal pole, and the posterior boundary was defined by the slice anterior to the trigone of the lateral ventricles. The superior boundary of the temporal lobe was demarcated by the Sylvan fissure, and the lateral and posterior boundaries by the outline of the superior, middle, and inferior gyri. The medial temporal lobe boundary was defined by drawing a line perpendicular to the axis of the temporal stem from the inferior aspect of the insula around medial temporal lobe structures. The anterior slice of the superior temporal gyrus began at the temporal stem, and the most posterior slice was defined by the slice anterior to the trigone of the lateral ventricles. The superior temporal gyrus was bounded superiorly by the Sylvan fissure, inferiorly by the superior temporal sulcus, laterally by the gyral outline, and medially by a line connecting the superior temporal sulcus to the circular sulcus. Interrater reliability for the temporal lobe and superior temporal gyrus was based on 20 area measurements of each region conducted by two raters (N.L.B. and R.W.B.). The intraclass correlation coefficients were greater than 0.95 for both structures.
Morphometric analyses were performed with the use of the Loats image analysis system
(44). The sample function of the system was used to determine volumes of the temporal lobe, superior temporal gyrus, caudate, and amygdala/hippocampus. This function enables the investigator to outline the region of interest directly on the computerized MRI image and calculates the area of the demarcated region. The threshold function of the system was used to determine volumes of prefrontal gray and white matter. This function enables the investigator to partition gray matter from both CSF and white matter by assigning nonoverlapping signal intensity ranges to each tissue compartment. The threshold function was not used to partition the temporal lobe and superior temporal gyrus into gray and white matter components because of problems with radio frequency inhomogeneity.
A three-way repeated measures analysis of covariance was performed on all measures, with gender and diagnostic group (comparison subjects versus schizophrenic patients) as between-group factors and hemisphere (right versus left) as a within-group factor. Age at the time of MRI scanning and total cranial volume were used as covariates to correct for differences in age, duration of illness, and head size (
table 1). Post hoc analysis was performed using the Bonferroni method. Chi-square tests and t tests were used to compare total cranial volume, demographic variables, and clinical variables of the patients and comparison subjects. Volumes of the superior temporal gyrus and amygdala/hippocampal complex were excluded from temporal lobe volume to prevent redundancy within statistical analyses. Prefrontal cortex total volume and volumes of the prefrontal cortex gray and white matter and caudate were used to assess the specificity of temporal lobe results.
RESULTS
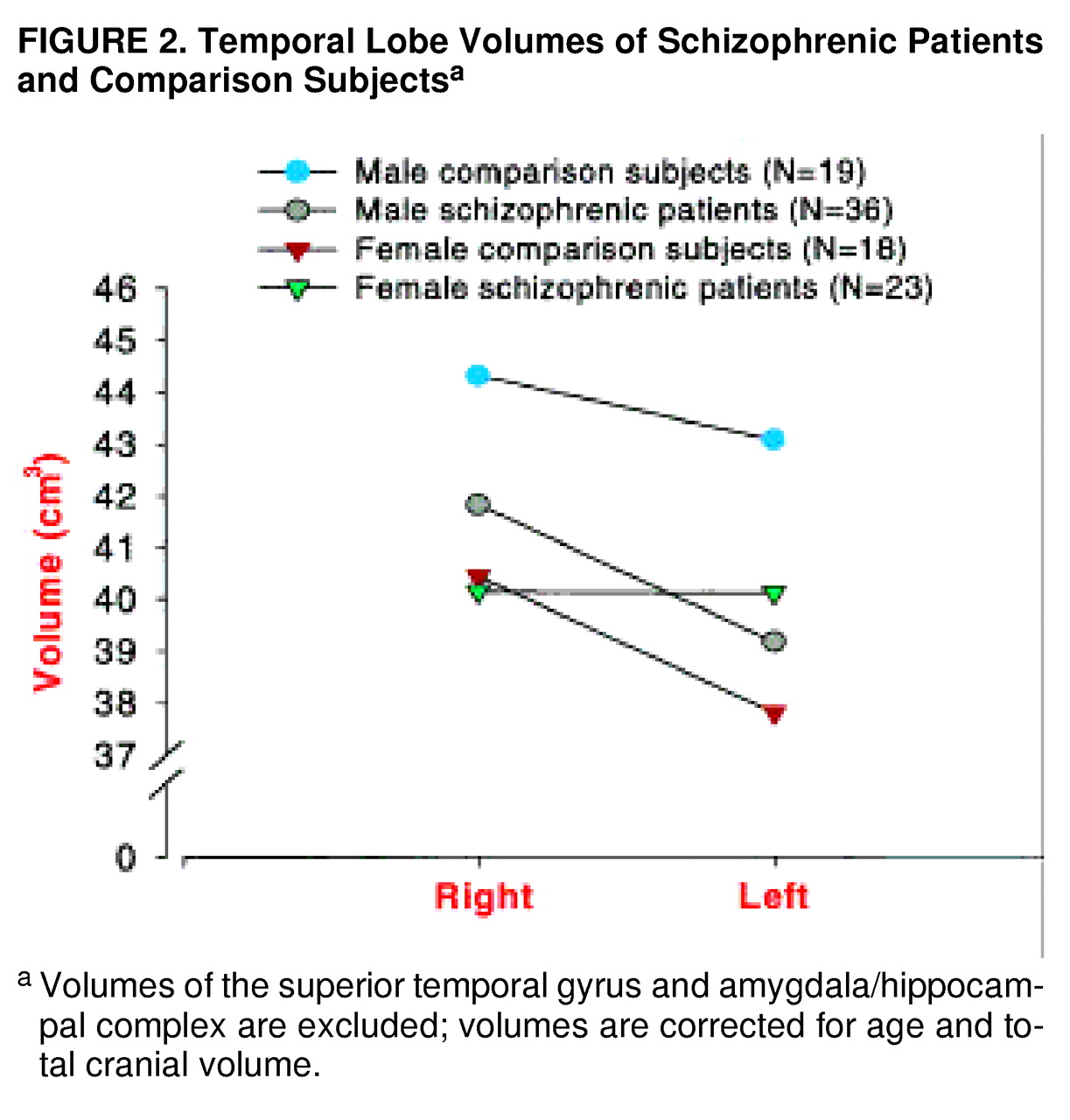
For temporal lobe volume (
table 2,
figure 2), there was not a significant main effect of diagnosis (F=1.79, df=1, 90, p=0.18), but there was a significant main effect of gender (F=6.50, df=1, 90, p=0.01). There was a significant three-way interaction (gender by diagnostic group by hemisphere). The male patients had significantly smaller volumes on the left than the male comparison subjects (t=3.2, df=182, p<0.01, Bonferroni post hoc test). There were no significant differences between the female patients and female comparison subjects; however, the female patients demonstrated nonsignificantly greater left temporal lobe volume.
For superior temporal gyrus volume (
table 3), there was a significant main effect of diagnosis. The patients with schizophrenia had significantly smaller volumes bilaterally than the comparison subjects. There were no statistically significant interactions. There was a significant hemisphere effect, with right volume greater than left volume in both diagnostic groups (F=10.97, df=1, 92, p=0.001).
For amygdala/hippocampal complex volume (
table 3), there was a significant main effect of diagnosis. The patients with schizophrenia had significantly smaller volumes bilaterally than the comparison subjects. There were no statistically significant interactions, and there was no significant hemisphere effect for either diagnostic group (F=0.24, df=1, 92, p=0.63).
For prefrontal cortex total volume (
table 3), there was a significant main effect of diagnosis. The patients with schizophrenia had significantly smaller volumes bilaterally than the comparison subjects. There were no statistically significant interactions. There was a significant hemisphere effect, with right volume greater than left volume in both diagnostic groups (F=12.49, df=1, 91, p=0.005).
For prefrontal cortex white matter volume (
table 3), there was a significant main effect of diagnosis. The patients with schizophrenia had significantly smaller volumes bilaterally than the comparison subjects. There were no statistically significant interactions. There was a significant hemisphere effect, with left volume greater than right volume in both diagnostic groups (F=39.70, df=1, 89, p=0.005).
For prefrontal cortex gray matter volume (
table 3), there was no significant main effect of diagnosis (F=0.48, df=1, 89, p=0.49). There was a significant hemisphere effect, with right volume greater than left in both diagnostic groups (F=132.23, df=1, 89, p=0.005). There were no statistically significant interactions.
Caudate volume (
table 3) was significantly larger on the left in the patients than in the comparison subjects (F=5.60, df=1, 88, p<0.02), and there was a nonsignificantly greater volume on the right in the patients with schizophrenia versus the comparison subjects. There were no statistically significant interactions. There was a significant effect of hemisphere.
DISCUSSION
These results demonstrate that in our outpatient clinic population, abnormal volumes of the temporal lobe were evident in the patients with schizophrenia in comparison with the healthy subjects. There was a significantly smaller left temporal lobe volume in the male patients than in the male comparison subjects, but there was no significant difference in temporal lobe volume between the female patients and the female comparison subjects. The latter finding is consistent with the report of Cowell et al.
(19). The male and female patients had smaller volumes of the amygdala/hippocampal complex and superior temporal gyrus as compared with the male and female comparison subjects. There were no gender differences in these structures. These findings have been reported by some, but not all, previous MRI studies
(18,
21,
22,
24–
27,
29).
Prefrontal cortex total volume, volumes of the prefrontal cortex gray matter and white matter, and caudate volume were important for determining the specificity of the influence of gender on temporal lobe volume in the patients versus the comparison subjects. There were no significant gender interactions for these structures. Prefrontal cortex white matter and total volumes were significantly smaller bilaterally in the male and female patients than in the comparison subjects, which was reported in an earlier study that used a smaller study group
(25). The caudate was significantly larger on the left in the male and female patients than in the male and female comparison subjects, and there was a nonsignificant increase on the right in the patients versus the comparison subjects. This finding was also reported in the earlier study with a smaller study group
(25). Taken together, these results suggest that the influence of gender on abnormal structure in patients with schizophrenia is limited to the temporal lobe, an effect that is not localized to either the superior temporal lobe or the amygdala/hippocampal complex.
The female patients were significantly older than the female comparison subjects, the male patients, and the male comparison subjects. There was also a significantly greater duration of illness among the female patients as compared with the male patients (
table 1). Age at the time of MRI scanning was used as a covariate to correct for these differences. There were no other significant differences in demographic variables.
We were unable to differentiate volumes of the temporal lobe and superior temporal gyrus gray and white matter. This would have been beneficial in assessing whether group differences were related to alterations in gray matter volume, white matter volume, or both. Several studies have reported significant gray matter abnormalities in temporal lobe structures in schizophrenic subjects
(20,
24,
27,
28,
42). The majority of these gray matter abnormalities, however, have been reported in male patients. The extent of the presence of these abnormalities in female patients remains to be determined.
The results of the current study suggest that gender does not exert a major influence on volumes of the superior temporal gyrus or the amygdala/hippocampal complex. The finding of volumetric differences in the temporal lobe (excluding volumes of the superior temporal gyrus and amygdala/hippocampal complex) suggests that there may be gender-specific abnormalities in the middle temporal gyrus, the inferior temporal gyrus, and/or the fusiform gyrus in male and female patients with schizophrenia. Positron emission tomography (PET), electrical stimulation, and electrophysiologic studies provide evidence that the middle temporal, the inferior temporal, and the fusiform gyri are important in performing several complex cognitive and sensory integrative functions. PET studies have shown that the left middle temporal gyrus and inferior temporal gyrus are important components in language processing, and the right middle temporal gyrus is an important component in tonal memory in normal subjects
(45,
46). Studies of aphasic patients have shown that the posterior middle temporal gyrus and inferior temporal gyrus, along with the inferior parietal lobule, may play an important role in auditory comprehension, naming, oral reading, and repetition
(46–
48). Electrical stimulation studies of the inferior temporal and the fusiform gyri have been shown to result in significant impairments in language production
(49,
50). Suzuki et al.
(51), using intracranial EEG, demonstrated a direct connection between the left fusiform gyrus and Wernicke’s area. Visual evoked potential and clinicopathologic studies suggest that the fusiform and the inferior temporal gyri are also involved in color perception, color integration, visuospatial processing, and facial recognition
(52–
54). Patients with schizophrenia demonstrate substantial difficulties with many of these cognitive processes, and it seems likely that volumetric increments or decrements in the middle temporal, the inferior temporal, or the fusiform gyri may potentially reflect some of these difficulties and, in turn, identify specific neuroanatomical substrates of cognitive pathology based on gender.
Future studies will focus on examining these structures by using more advanced MRI volumetric techniques with three-dimensional images to correlate morphology with neuropsychological testing and clinical symptoms.