There is substantial evidence from animal research that neurotransmitter systems, which are affected by antipsychotic drugs, are also involved in sexual functions. Hypothalamic dopamine, for instance, inhibits the release of pituitary prolactin and, therefore, can enhance sexual drive
(1). On the other hand, serotonin increases the release of prolactin, thereby potentially reducing libido
(2,
3). In addition, antipsychotics can influence neurotransmitters mediating sexual functions at the end organ site
(4). Stimulation of the cholinergic system results in vasodilatation, by which the corpora cavernosa become filled with blood, but sympathic stimulation produces vasoconstriction, which leads to an inhibition of erection
(5).
Given the different receptor-blocking profiles of antipsychotic drugs, distinct sexual side effects would be expected. Despite the fact that sexual side effects are discussed as one of the most important factors influencing compliance, only a few investigators have studied frequency and course of these side effects
(6).
METHOD
From 1989 to 1996 all patients who received clozapine or haloperidol on the inpatient units of the Department of Psychiatry of Innsbruck University Clinics were investigated prospectively in a drug monitoring program. Sexual side effects were regularly assessed with the Udvalg for Kliniske Unders�gelser (UKU) Side Effect Rating Scale, an observer-rated scale
(7). All patients gave informed consent after a full explanation of the procedure by a physician.
For the purpose of subsequent statistical analysis, patients with a score of 1 or higher on any of the relevant items of the UKU Side Effect Rating Scale at any time during treatment were considered as having sexual side effects. We analyzed only those side effects for which raters had decided that the relation of the symptom to drug treatment was possible or probable. Ratings were performed weekly during the first 6 weeks of treatment and monthly thereafter. All patients suffered from schizophrenia or schizophreniform disorder (DSM-III-R). Compliance was regularly assessed by clinical interviews and plasma level monitoring.
Two measures were used to assess sexual disturbances and their time course. The course of sexual disturbances (comparison of sexual disturbances during weeks 1–3 and weeks 13–18) was evaluated by using Wilcoxon’s matched pairs test. Occurrences of sexual disturbances until week 6, recorded as dichotomous variables (occurred versus not occurred) in the two treatment groups, were compared by using the chi-square test.
The effects of dose and plasma level on sexual disturbances (occurrence within the first 6 weeks) were studied by using a logistic regression analysis; this method was also used to check for a possible effect of asthenia, sedation, and other medications (benzodiazepines, anticholinergic drugs, β-blockers, antidepressants, and anticonvulsants). The impact of sexual disturbances on compliance was studied by using the chi-square test. Noncompliance was defined as a discontinuation of medication by the patient against the recommendation of the treating physician.
RESULTS
We investigated 153 patients; 53 (41 men and 12 women) were treated with haloperidol, and 100 (75 men and 25 women) were treated with clozapine. Mean age (haloperidol group: mean=26.4 years, SD=8.4; clozapine group: mean=28.6, SD=9.5) and duration of illness (haloperidol group: mean=37.8 months, SD=61.6; clozapine group: mean=42.5 months, SD=54.6) were comparable in both groups (Mann-Whitney U test), as was sex distribution (chi-square test). The mean dose of haloperidol was 16.0 mg/day (SD=11.8) (mean plasma level=11.4 ng/ml, SD=15.2), and the mean dose of clozapine was 260.7 mg/day (SD=139.0) (mean plasma level=183.3 ng/ml, SD=150.2).
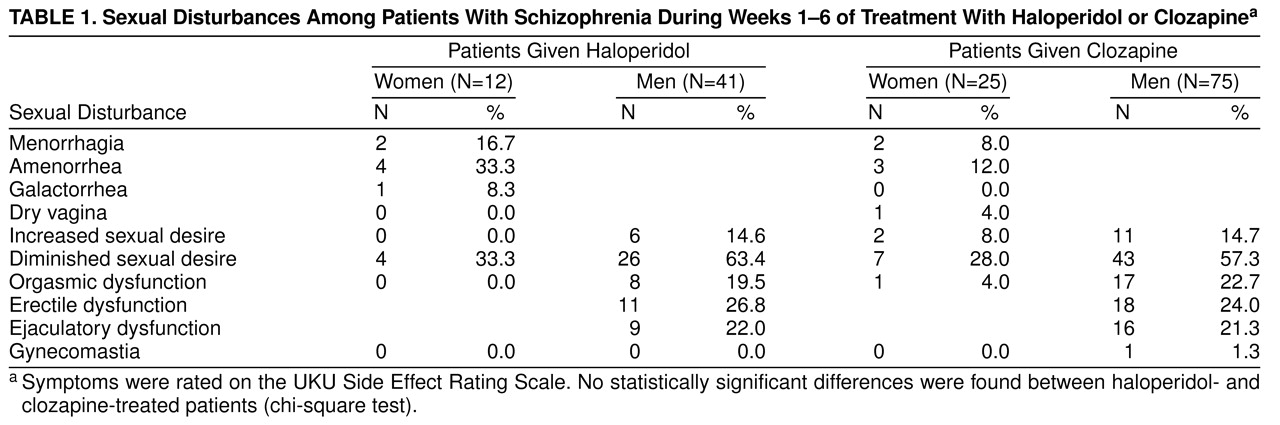
Table 1 shows the frequency of sexual disturbances with clozapine and haloperidol treatment during weeks 1 to 6. For the male patients, the nonsignificant differences between the two neuroleptic medications go along with estimates of a relative risk close to 1, indicating that group differences were in fact small (relative risk for diminished sexual desire for haloperidol versus clozapine=1.10, 95% confidence limits=0.81–1.49; relative risk for functional disturbances=1.17, 95% confidence limits=0.63–2.18). For the female patients, it cannot be ruled out that the nonsignificant results may be a consequence of the small number of patients (N=37).
Over the first 18 weeks of clozapine treatment, sexual disturbances (impaired sexual desire) decreased from 53.4% to 22.2% among the male patients (z=4.42, N=36, p<0.001, Wilcoxon matched pairs test) and from 23% to 0% among the female patients (z=1.83, N=11, p=0.07, Wilcoxon matched pairs test). All male functional disturbances (erectile and ejaculatory dysfunction) disappeared within the first 18 weeks of treatment. In contrast, menstrual disturbances remained basically unchanged during the first 18 weeks.
Among the male patients taking clozapine (N=75), there was a significant influence of plasma levels on diminished sexual desire (χ2=5.18, df=1, p=0.02, logistic regression, –2 log likelihood ratio) and functional disturbances (χ2=7.03, df=1, p=0.008, –2 log likelihood ratio). This was not found among the patients taking haloperidol (N=53) or in the female patients taking clozapine (N=25), which may be a consequence of the small number of patients in this group. In contrast, dose had no influence on sexual dysfunctions in male and female patients taking either medication. Furthermore, sedation, asthenia, and taking other medications were not associated with any of the sexual adverse events.
A significantly higher percentage of noncompliant patients was found in the haloperidol group (χ2=10.83, df=1, p=0.001). It is surprising that neither functional disturbances nor diminished sexual desire showed any influence on compliance in either group.
DISCUSSION
Clozapine has been thought to be associated with fewer sexual side effects because of its weaker blockade of D
2 receptors and because, in contrast to classical antipsychotics, clozapine has only a minimal effect on plasma prolactin levels
(4). We have found comparable rates of sexual side effects for clozapine and haloperidol.
The rates of adverse sexual events appear to be lower in female patients, as reported in the few published studies dealing with this topic
(8). In spite of these findings, which are corroborated by our study, it is likely that men and women are equally affected by sexual side effects. The lower reported rate of sexual disturbances in women could be explained by the fact that the women in our investigation might have been more guarded than the men when speaking about sexual disturbances. Additionally, the judgment about erectile function is much easier than, for instance, the determination of vaginal lubrication or the quality of an orgasm.
We found an influence of clozapine plasma levels on sexual disturbances in male patients. This is important given the different dose and plasma level recommendations for clozapine in the United States and Europe
(9).
Although sexual disturbances are often discussed as a significant reason for noncompliance, we could not demonstrate a correlation between sexual disturbances and compliance or lack of it. Elsewhere
(10), we hypothesized that regularly discussing adverse effects with patients, as well as subsequently treating them, if possible, might be responsible for the fact that adverse events had no negative influence on compliance in our program.