Changes in weight associated with depression are complex phenomena that are likely influenced by a number of disease-specific factors, including alterations in appetite and activity, as well as factors specific to individual antidepressant medications. Several reports have assessed these interactions during acute and extended treatment with tricyclic antidepressants
(1–
3) and lithium
(3–
5) and suggest that these agents are associated with weight gain, although data beyond acute treatment generally come from small, poorly controlled studies. Selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine are similar in efficacy and better tolerated than these older agents and have become the most common therapy for depression. Although acute therapy with fluoxetine is not associated with greater weight gain than placebo and in some studies is associated with weight loss
(6,
7), controlled studies of weight changes in successfully treated patients who continue therapy with fluoxetine or other SSRIs over longer periods have not been reported. As a result, published data concerning the relationship of SSRIs and weight change beyond the period of acute therapy are limited to uncontrolled or anecdotal reports
(8).
METHOD
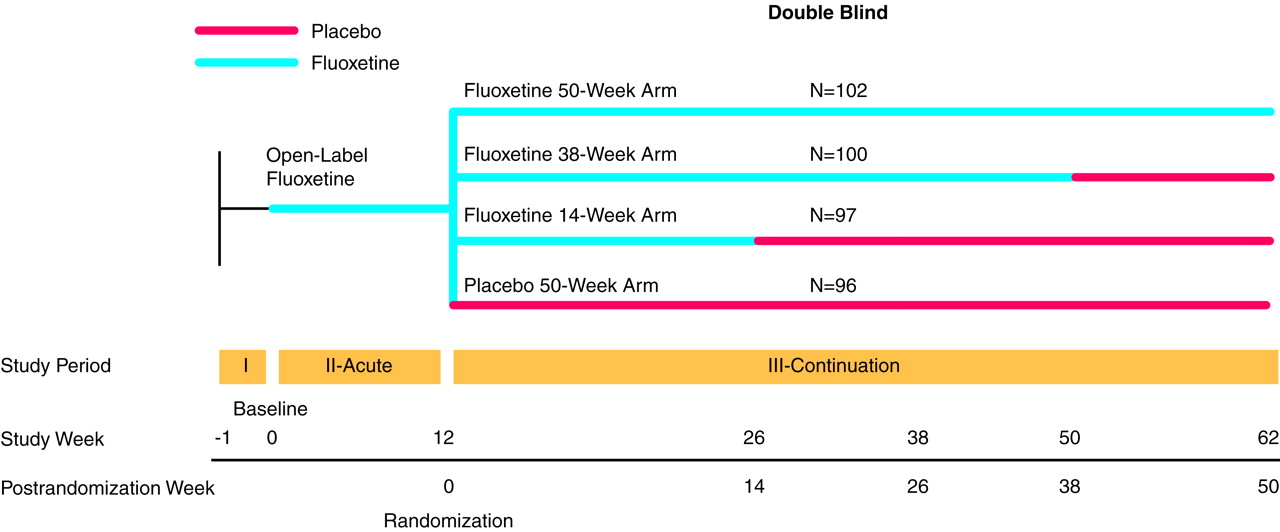
The overall design of this study has been reported elsewhere
(9) and will be summarized briefly here. The study assessed the efficacy of fluoxetine in preventing depression relapse following successful acute treatment. The study was conducted at five academic centers in the United States. After receiving a complete description of the study, all patients gave written informed consent. Outpatients who met the criteria for a major depressive episode (defined as meeting DSM-III-R criteria with a modified 17-item Hamilton Depression Rating Scale
(10) score of 16 or greater [the Hamilton depression scale was modified to include questions assessing weight gain, appetite increase, and hypersomnia]), who met entry criteria and agreed to participate, were treated for 12 weeks with open-label fluoxetine, 20 mg/day. Following acute treatment, patients whose illness had remitted (as identified by a modified Hamilton depression scale score of 7 or less) were randomly assigned to continuation treatment with either placebo; 14 weeks of fluoxetine, 20 mg/day; 38 weeks of fluoxetine, 20 mg/day; or 50 weeks of fluoxetine, 20 mg/day (
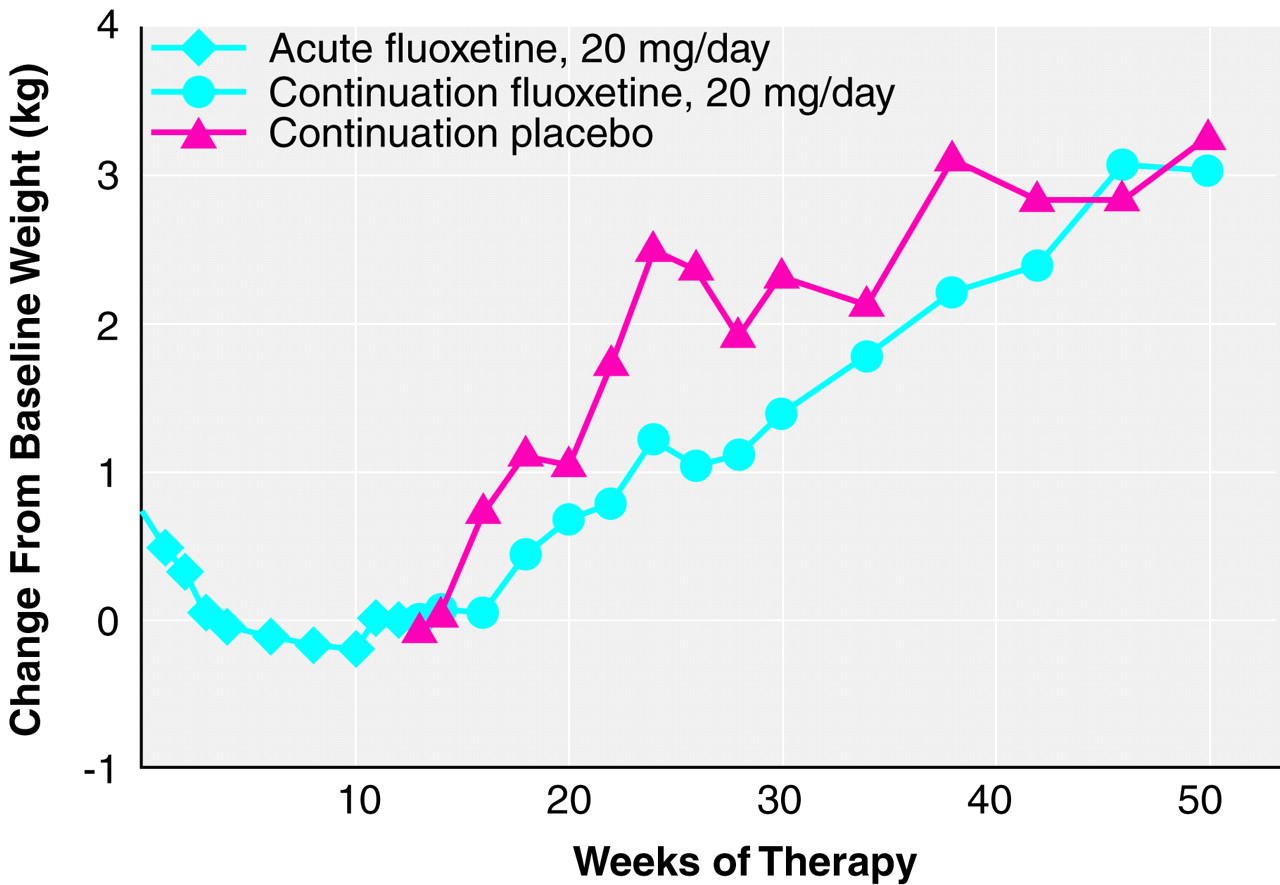
Figure 1). Height was measured at the initial visit, and weight was assessed at every visit. Changes in weight were examined for both the acute phase of the trial and during continuation treatment for patients whose depressive symptoms had remitted. Results are given as means and standard deviations.
To assess the relationship between acute fluoxetine treatment and weight, we examined change in weight (mean absolute change in kilograms, mean percentage change, and number of patients who experienced a 7% or greater increase in weight—considered the standard of extreme weight gain in clinical trials) from baseline (week 0) to random assignment (week 12). All data from patients receiving treatment were analyzed by using a last-observation-carried-forward analysis. In addition, the subgroup of patients who completed 12 weeks of treatment and whose depressive symptoms remitted was analyzed. We also assessed visit-wise change in weight from baseline (week 0) to random assignment (week 12) for those whose depression had remitted and who eventually completed 50 weeks of treatment.
To assess the relationship between longer-term treatment and weight, we analyzed weight change (mean absolute change in kilograms, mean percentage change, and number of patients who experienced a 7% or greater increase in weight) in fluoxetine- and placebo-treated patients from baseline (week 0) and random assignment (week 12) to completion of 26, 38, or 50 total weeks of treatment (14, 26, or 38 weeks, respectively, of continuation treatment). These analyses were repeated for all patients by using last-observation-carried-forward analysis. Additional analyses assessed visit-wise change in weight from random assignment (week 12) to end point for patients receiving fluoxetine or placebo who completed 38 weeks of continuation treatment (50 total weeks of treatment). Finally, we assessed the relationship between weight change over 50 weeks (12 weeks of acute treatment plus 38 weeks of chronic therapy) and 1) initial body mass index, 2) initial appetite loss at baseline (derived from the score of the relevant items on the modified Hamilton depression scale at study entry), and 3) change in appetite over the course of the study (derived from assessing a change in score on the relevant items of the modified Hamilton depression scale).
All comparisons were evaluated in a two-tailed fashion at the 0.05 level for statistical significance. Patient baseline characteristics were compared between patients in the fluoxetine and placebo groups who completed 26, 38, and 50 total weeks of treatment as well as for all patients in continuation treatment by using two-sample t tests for continuous variables and Pearson’s chi-square tests for discrete variables. Weight change during the acute treatment period was assessed for all patients with baseline and post-baseline measurements by using a last-observation-carried-forward analysis and t test. This analysis was repeated for all patients who completed 12 weeks of therapy and met remission criteria. Visit-wise change in weight during the same period was assessed by fitting a mixed-effects model for repeated measures data with visit as the within-subject factor by using an autoregressive covariance matrix.
Change in weight (mean absolute change in kilograms and percentage change from weight at the time of study entry and the time of random assignment) between the fluoxetine and placebo treatment groups during the continuation period to each of the end points (26, 38, and 50 total weeks) was compared by using a last-observation-carried-forward analysis; change in weight for patients completing the study was analyzed with two-sample t tests. For the same groups, the proportion of patients with 7% or greater weight gain at each time point was compared by using Pearson’s chi-square analysis. For the group of patients who completed 50 total weeks of treatment, a mixed-effects model with body mass index as covariate, visit as the within-subject factor, treatment as the between-subject factor, and a treatment-by-visit interaction using an autoregressive covariance matrix was fitted to the repeated measures data on weight to see if there was a time effect or an interaction effect of time-by-treatment on weight during continuation therapy. The relationship between initial body mass index and weight change for patients who completed 50 total weeks (12 weeks of acute fluoxetine treatment plus 38 weeks of fluoxetine or placebo therapy) was also assessed by correlation analysis by using Pearson’s product moment correlation. With the same patients, the association between initial appetite loss (derived from score on the relevant items of the modified Hamilton depression scale) and weight change over 50 total weeks and the association between change in appetite over the course of the study (derived from a change in score on the relevant items of the modified Hamilton depression scale) and weight change were analyzed by using Pearson’s product-moment correlation.
RESULTS
Weight data were available for 839 patients entered into the open-label, acute therapy phase; 395 patients who met remission criteria were randomly assigned to a continuation therapy group. Efficacy outcome measures showed fluoxetine to be superior to placebo in preventing the relapse of depression. These results have been reported elsewhere
(9).
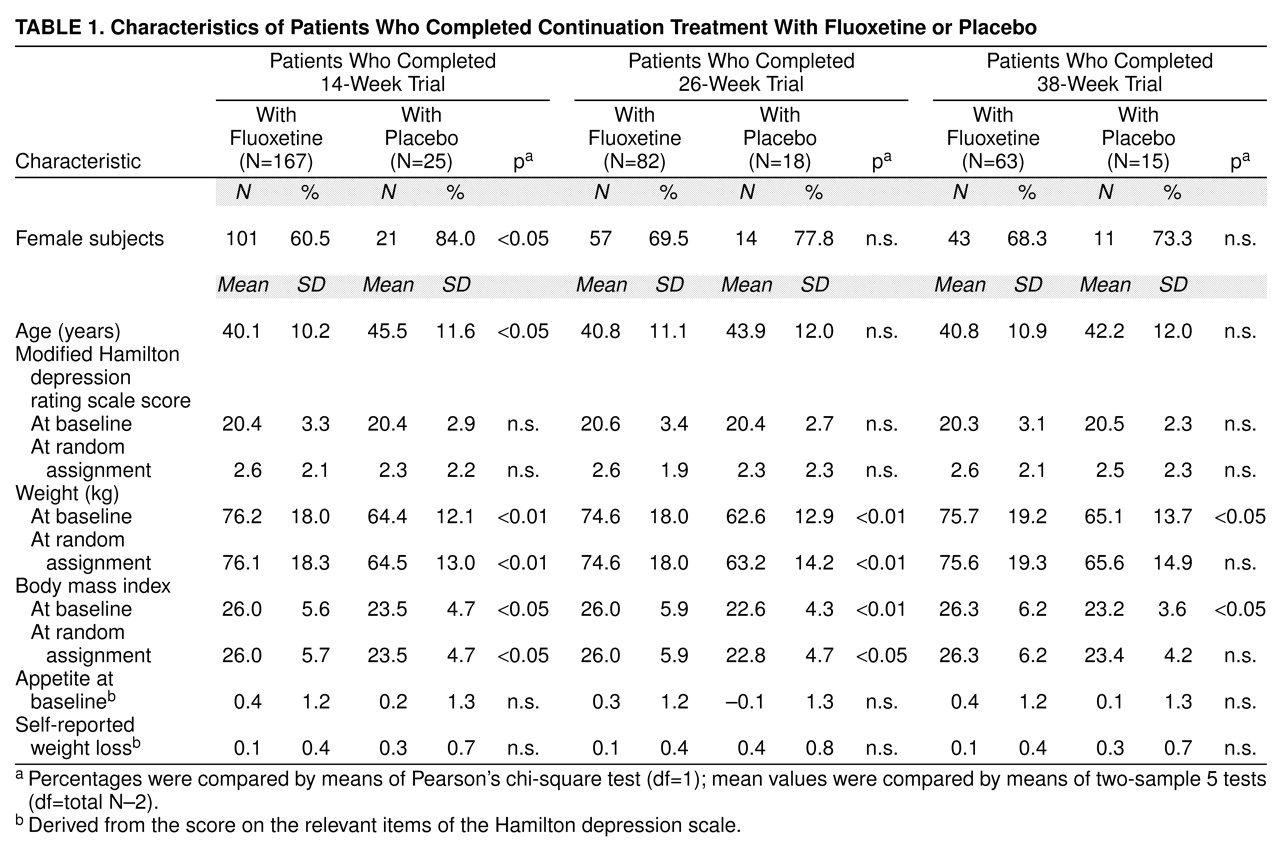
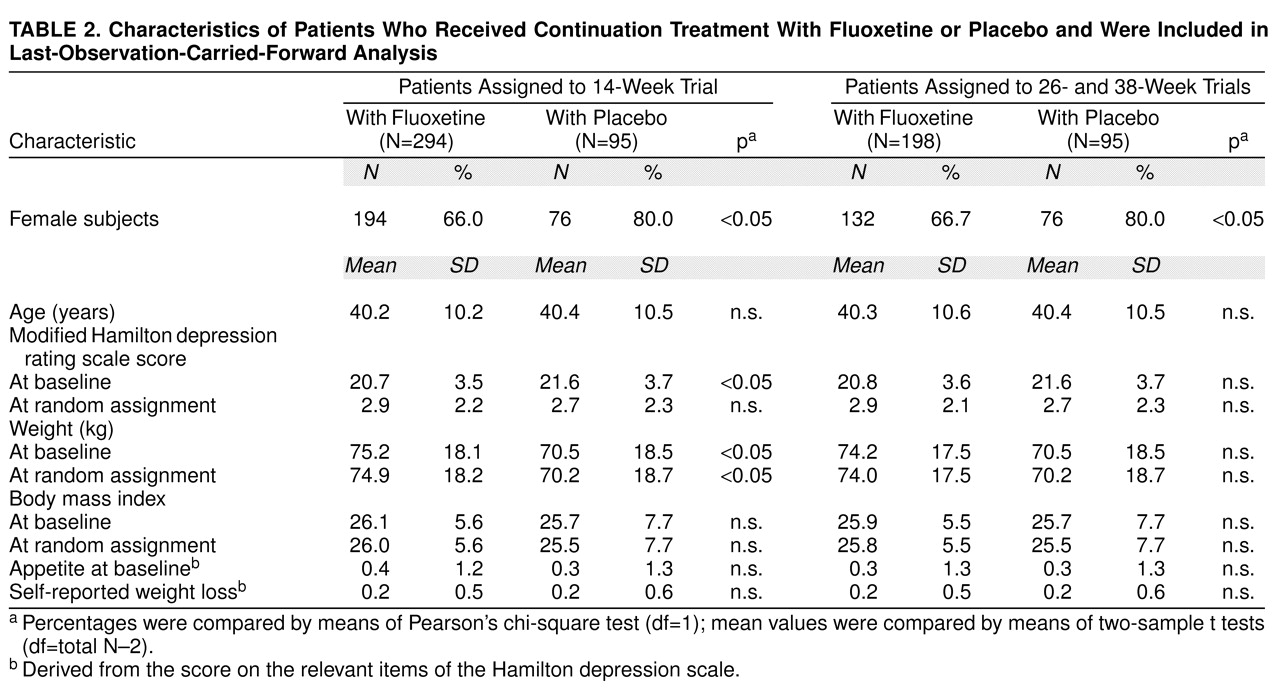
Baseline variables were similar for all patients assigned to a continuation treatment group. In the subgroup of patients who completed 14 weeks of continuation treatment, the fluoxetine group was younger and had a lower percentage of women than the placebo group. In the subgroups of patients who completed 14, 26, or 38 weeks of continuation therapy, the patients receiving fluoxetine treatment were heavier at study entry and had a higher mean body mass index than patients receiving placebo. There were no significant differences among groups in modified Hamilton depression scale mean scores at study entry or time of random assignment, and there were no differences in self-reported weight loss or appetite before study entry. According to last-observation-carried-forward analysis, there was no imbalance in baseline body mass index (all subgroups) or baseline weight (26- and 38-week subgroups). Patient characteristics are summarized in
Table 1 and
Table 2.
Acute Phase
At least two observations of weight were available for 832 patients. Results are presented as mean kg. During the 1-week period following study entry but before starting fluoxetine treatment, there was a small but statistically significant weight gain (N=801; initial weight=73.5 kg, SD=17.7; weight at start of drug therapy=73.8 kg, SD=18.0) (t=3.2, df=800, p<0.01, paired t test). For all patients (N=832, last-observation-carried-forward analysis, baseline to end point), there was a small but statistically significant decrease in weight during the 12-week acute therapy period (weight at baseline=73.5 kg, SD=17.8; weight at end point=73.2 kg, SD=18.1) (mean=–0.35, SD=3.5; t=–2.9, df=831, p<0.01, paired t test). An examination of the data from those patients who met remission criteria at the end of the acute phase and had at least two observations of weight available (N=403) yielded similar but not statistically significant results (weight at baseline=73.9 kg, SD=18.6; weight at end point=73.5 kg, SD=18.3) (mean=–0.26, SD=3.3; t=–1.6, df=402, p=0.11, paired t test). Weight change during acute therapy was similar for all patients in continuation treatment at week 12, regardless of eventual group assignment. Likewise, weight change during the acute period was similar for patients who ultimately completed 26, 38, or 50 total weeks of continuation treatment with fluoxetine or placebo, regardless of group assignment. Visit-wise weight change for patients whose illness remitted and who completed 38 weeks of continuation treatment showed that the acute weight loss occurred primarily during the initial 4 weeks of therapy, with stabilization of weight thereafter (
Figure 2).
Continuation Therapy
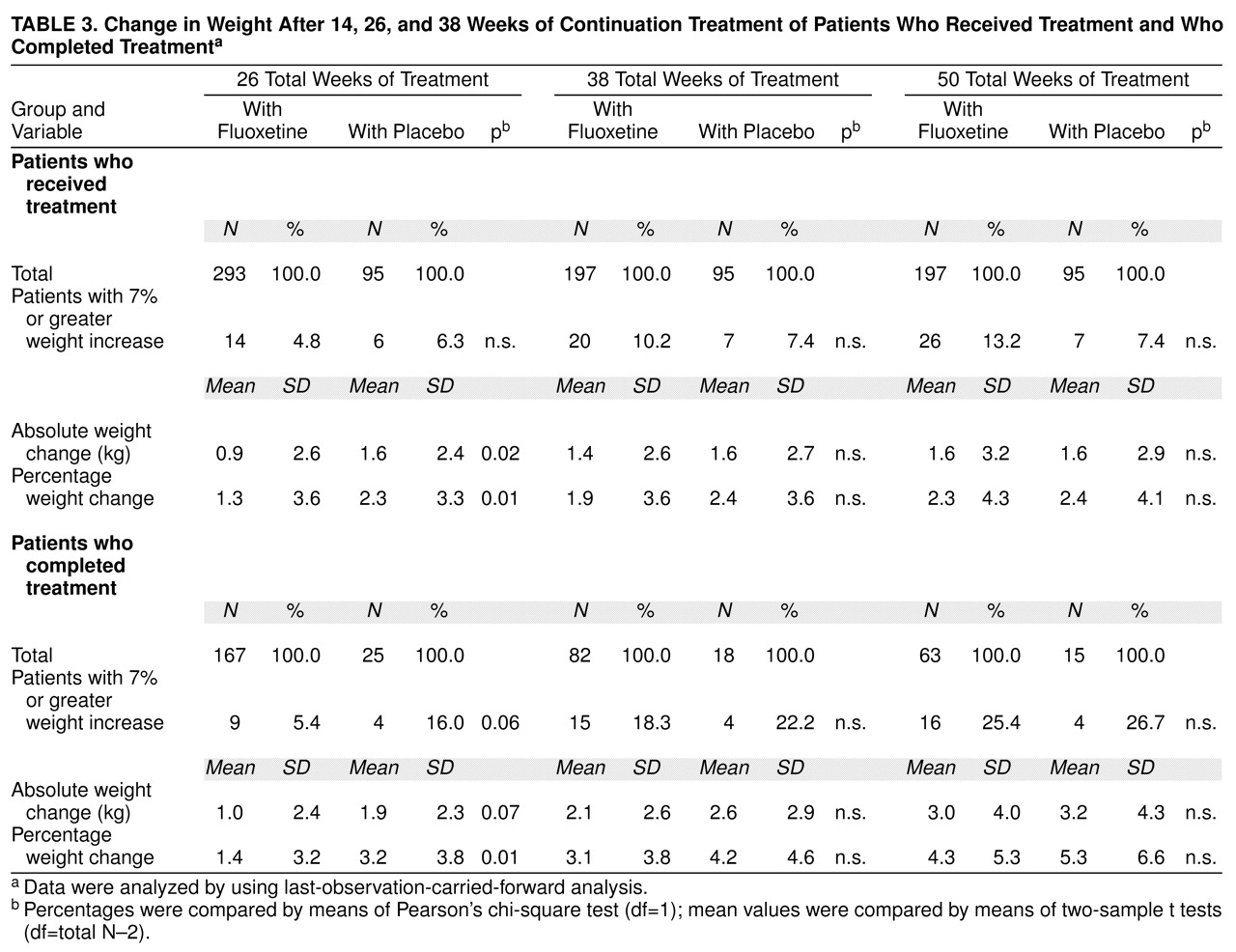
Patients completing 26, 38, or 50 total weeks of treatment. There was a statistically significant weight gain from random assignment (week 12) to end point among all patients (fluoxetine and placebo groups) who completed each interval (12 weeks of acute treatment and 14, 26, or 38 weeks of continuation therapy). Mean absolute weight change at week 26 was 1.1 kg (SD=2.4) (t=6.3, df=191, p<0.001, paired t test; N=192); at week 38, 2.2 kg (SD=2.7) (t=8.3, df=99, p<0.001, paired t test; N=100); and at 50 weeks, 3.1 kg (SD=4.1) (t=6.7, df=77, p<0.001, paired t test; N=78). Reanalysis among the same patients using weight from study entry to the same end points yielded similar results.
Comparison between patients receiving continuation treatment with fluoxetine and patients receiving placebo. During the continuation treatment phase, patients who received 26 total weeks of fluoxetine treatment (12 weeks of acute treatment and 14 weeks of continuation treatment) gained slightly less weight as a percentage of weight at random assignment than did patients who received 12 weeks of fluoxetine therapy followed by 14 weeks of placebo (
Table 3), but weight gain (either change in absolute kg or change as a percentage of initial weight) during the continuation phase did not vary between the two treatment groups after either 38 or 50 total weeks of treatment. Similarly, at the end of 26 total weeks of treatment, the mean absolute change in weight and the percentage of patients with a 7% or greater gain from their initial weight were higher, though not statistically significant (t=–1.9, df=190, p=0.07, two-sample t test; t=–1.9, df=192, p=0.06, two-sample t test, respectively), in the placebo group compared with the fluoxetine group, whereas at 38 and 50 weeks, both groups were similar. Reanalysis using last-observation-carried-forward analysis that included all patients receiving continuation treatment yielded similar results, except that at 26 weeks of treatment, the mean absolute weight change was statistically significantly higher in the placebo group (t=–2.3, df=386, p=0.02, two-sample t test), whereas the percentages of patients with a 7% or greater gain from their initial weight were comparable between groups (
Table 3). Weight change did not vary among patients assigned to receive placebo who dropped out before completing 26 total weeks (12 weeks of acute treatment and 14 weeks of continuation therapy) compared with patients receiving placebo who completed 26 total weeks. Visit-wise analysis of weight change among all patients who completed 50 total weeks (12 weeks of acute and 38 weeks of continuation therapy) showed a statistically significant weight gain over the course of continuation therapy (F=3.89, df=15, 1068, p<0.001, mixed effects model), and this effect was similar in both treatment groups (F=1.87, df=1, 76, p=0.18, mixed effects model). Adjusting for the initial imbalance in body mass index did not change the outcome.
Relationship between initial weight or appetite and change in weight. Initial body mass index and weight change were not statistically significantly correlated at any time point for all patients or for either treatment group. For all patients completing 50 total weeks of therapy, as well as among those receiving fluoxetine at week 12 who completed 50 total weeks of therapy, greater initial appetite loss was associated with greater weight gain at the end of the study (all patients: r=0.28, N=78, p=0.01; fluoxetine group: r=0.28, N=63, p=0.02; placebo group: r=0.25, N=15, p=0.37). For the same two groups, improvement in appetite over the course of the study (as assessed by a change in score on the appetite items of the Hamilton depression scale) was statistically significantly correlated with weight gain over 50 weeks and approached statistical significance for patients receiving placebo (all patients: r=0.31, N=78, p=0.006; fluoxetine group: r=0.25, N=63, p=0.05; placebo group: r=0.46, N=15, p=0.08).
DISCUSSION
Patients whose depressive symptoms remitted during acute treatment with fluoxetine who were then randomly assigned to continuation treatment with either fluoxetine or placebo experienced a mean weight gain of approximately 3 kg during 50 weeks of continuation therapy that was not related to treatment assignment. Acute treatment with fluoxetine was associated with a modest (less than 0.5 kg) weight loss that occurred primarily during the initial 4 weeks of therapy.
Several studies have assessed change in weight with long-term tricyclic antidepressant therapy and suggest that weight gain is a specific effect of these agents
(1–
3). We are not aware of any reports of controlled studies examining weight change during long-term therapy with fluoxetine or other SSRIs in depressed patients. A recent report suggested that fluoxetine and other SSRIs may be associated with extreme weight gain during extended therapy
(8). However, many nondrug factors can influence weight gain during and after recovery from depression, and that report, based on anecdotal, unsystematically collected data, must be treated with caution. The current study differs from previous reports in its inclusion of a placebo, in the large number of patients studied, and in the systematic collection of weight data and the standardization of treatment conditions. The data reported here suggest that daily therapy with fluoxetine, 20 mg, is not associated with greater weight gain than placebo during a total treatment period of up to 50 weeks (12 weeks of acute therapy and 38 weeks of continuation therapy) and that during extended fluoxetine treatment, observed weight gain is associated with recovery from depression rather than with fluoxetine treatment
(11–
13).
Several factors limit the interpretation of these data. Although these patients were observed for up to 50 weeks of treatment, outcomes could change over longer periods. All patients entering the continuation phase of the study had received acute treatment with fluoxetine, and it is possible that the observed outcomes during continuation treatment were affected by previous acute treatment. In addition, all patients in this study were treated with fluoxetine, 20 mg/day, and different doses could be associated with different effects on weight. We also cannot rule out the possibility that a larger or longer study would show effects that were not observed here. Absence of even a trend toward differences between fluoxetine and placebo treatment at 1 year, however, suggests that any such effects would likely be small and of limited clinical importance. Finally, these findings represent outcomes in a relatively young and physically healthy population and may not generalize to other groups such as children or elderly patients
(14,
15).
There was a relatively small number of patients completing treatment in the placebo group because of the much higher rate of relapse among patients receiving placebo, particularly at the 38-week point. A small group could be unrepresentative of larger populations and could be a potential source of bias. This seems unlikely, however, since the results of the last-observation-carried-forward analysis (which includes all patients) were similar to those of the observed case analysis. Any bias in the last-observation-carried-forward-analysis would be expected to be toward greater weight gain in the fluoxetine group, which had a much lower return of depressive symptoms and hence less risk for weight loss during the continuation phase. Despite this potential bias, the last-observation-carried-forward-analysis and observed case analysis both showed no difference in weight change between the placebo and fluoxetine groups, suggesting that this finding is not an artifact of study group size.
Self-reported weight loss and severity of depression, both at entry into the study and at random assignment, were similar among the two groups; however, a potential concern is that among patients who completed each time period, the placebo group had a somewhat lower weight and body mass index at study entry than did the fluoxetine group. This imbalance appears to be because of the greater rate of relapse and the loss of more men in the placebo group, since, when all patients were considered, the groups were similar. (Note that the efficacy analysis did not suggest a differential treatment effect related to gender
[9].) Several factors, however, suggest that this imbalance did not significantly affect the results. First, baseline body mass index was included as a covariate in the analysis of those completing treatment and did not show a significant effect on the results. Second, changes in weight during the study were not correlated with initial body mass index; rather, predictors of change in weight were depression-related appetite changes at the outset of the study and improvement in appetite associated with recovery. Finally, the last-observation-carried-forward analyses, which, as already noted, showed similar results to the observed case analyses, showed no statistically significant difference among groups at baseline or random assignment for body mass index (all subgroups) or weight (26- and 38-week subgroups), and weight change at the last observation in placebo-treated patients who dropped out before 14 weeks of continuation treatment was similar to weight change in placebo-treated patients who completed 14 weeks of continuation treatment.
It is interesting that during the week following entry into the study but preceding the initiation of acute fluoxetine therapy, patients had a small but statistically significant weight increase. We cannot definitively account for this but speculate that patients may have experienced some nonspecific relief associated with entering treatment. The observed weight gain is consistent with previous reports that depressed patients tend to weigh less than matched healthy comparison subjects and that recovery from illness is associated with weight gain
(11).
The absence of a placebo during the acute therapy phase makes it impossible to dissociate illness and fluoxetine effects on weight during the initial 12 weeks of treatment. The acute phase results are, however, consistent with previous reports, which have generally found no change or modest weight loss during acute fluoxetine therapy. That this weight loss occurred during the initial 4 weeks of therapy is of interest from two perspectives: 1) this period is approximately the time by which marked symptom improvement is expected with fluoxetine treatment, and 2) 4 weeks is the point at which most patients achieve steady-state fluoxetine plus norfluoxetine levels. These observations suggest that factors related to both illness and acclimation to drug administration are relevant to the weight decreases observed during acute treatment.
Weight increases during the continuation phase were observed in both the placebo- and fluoxetine-treated groups, which is consistent with previous reports that the period following recovery from depression is associated with weight gain
(12,
13). It is likely that factors specific to depression, as well as other more general factors, account for this finding. In particular, recovery from depression is often associated with improved appetite and better social functioning, which could lead to greater food intake and potential weight gain. Less specifically, some people entering middle age may experience changes associated with the aging process that alter lifestyle and metabolic variables, potentially predisposing them to weight gain
(16,
17). The importance of illness-related factors and recovery from depression is further emphasized by the observed correlation between appetite and weight gain during the study. Patients who reported initial appetite loss tended to gain more weight over the course of the year, as did patients whose appetites increased from study entry to end point. This last effect was consistent for all patients considered together as well as for fluoxetine-treated patients and approached significance in the placebo group, despite its relatively small size, providing further evidence for the importance of depression-associated vegetative changes and their reversal during successful treatment.
This study provides no indication of a greater risk for extreme weight gain associated with fluoxetine, since the number of fluoxetine-treated patients with a 7% or greater increase in weight never exceeded that of patients in the placebo group. Further, change in weight appears to be normally distributed at all three time points (though it is perhaps slightly skewed to the right at 26 weeks). For each group, the number of patients whose weight increased by 7% or greater is also approximately the same as the number of patients expected at that distance from the mean. For example, at 14 weeks, a 7% increase in weight among the fluoxetine-treated patients is almost two standard deviations from the mean percentage change of 1.4%. The number of patients with a 7% increase in weight is 5.4%, close to the value predicted in a normal distribution. Similarly, at 26 weeks, a 7% increase in weight in the fluoxetine group represents approximately one standard deviation from the mean percentage weight change of 3.1%. The number of patients with a 7% or greater increase in weight is 17.9%, again close to the value predicted by a normal distribution. Thus, the approximately 18% of the patient population that had a weight gain of 7% or greater following 26 weeks of continuation therapy appears to be best accounted for by assuming that weight change is normally distributed rather than by any drug-specific factors.
In summary, these data provide evidence that after recovery from depression, patients are likely to experience modest weight gain, which increases over time. Fluoxetine may be associated with early weight loss and, compared with placebo, slower weight gain for periods up to 26 total weeks of treatment but does not appear to be associated with specific effects on weight during longer periods of therapy of up to 1 year.