Schizophrenia is a severe mental disorder affecting approximately 1% of the general population. The complex nature of schizophrenia is likely to represent the interplay between genetic vulnerability and environmental factors. Previous psychopharmacological studies have indicated that abnormal catecholamine neurotransmission may be important in the pathogenesis of schizophrenia
(1). Hence, genes for enzymes and receptors related to the biochemistry of catecholamine neurotransmission are considered reasonable candidate genes for schizophrenia.
Catechol
O-methyltransferase (E.C. 2.1.1.6) (COMT) is one of the enzymes that degrade catecholamine neurotransmitters
(2). Abnormal transmethylation of catecholamines by COMT, resulting in formation of psychotomimetic agents, such as mescaline, has been proposed to be associated with mental disorders
(3). Deletion of the COMT gene, mapped to 22q11, has been proposed to contribute to the overrepresentation of mental symptoms in patients with velocardiofacial syndrome, who have a microdeletion at 22q11
(4). Recently, the Schizophrenia Collaborative Linkage Group for Chromosome 22
(5) reported a susceptible locus for schizophrenia at 22q12. Taken together, these studies suggest that the COMT gene is a possible candidate gene for schizophrenia.
The genomic DNA of the COMT gene has been sequenced
(6), which should facilitate the identification of mutations of the COMT gene. In this study we tried to determine whether the COMT gene is a candidate gene for schizophrenia by systematically searching for mutations in schizophrenic patients. This study provided an opportunity to clarify the role of the COMT gene in schizophrenia.
METHOD
We recruited 177 Han Chinese patients (95 men and 82 women; mean age=47 years, SD=6) fulfilling the DSM-IV diagnostic criteria for schizophrenia from two private psychiatric hospitals in the Taipei area of Taiwan. As comparison subjects we recruited 99 Han Chinese adult nonpsychiatric outpatients (54 men and 45 women; mean age=45 years, SD=5) from a community hospital. Written informed consent was obtained. Genomic DNA was prepared from peripheral blood leukocytes.
The experiments were carried out in three stages. At the first stage, 50 patients were randomly selected for mutation analysis. Exons 1 and 2 were scanned by using single-stranded conformation polymorphism analysis, while exons 3 through 6 were examined by using an ABI autosequencer 373 (Perkin-Elmer, Washington, U.K.) to perform direct sequencing based on polymerase chain reaction (PCR).
At the second stage, PCR-based restriction genotyping methods were established, and the genotype of each subject was determined. An artificial HinfI restriction site was created for the genotyping of c.408C>G. The genotypes of c.472G>A, c.597G>A, and c.821-827insC were determined by treating the PCR products with restriction enzymes NlaIII, MspI, and BglI, respectively. The genotype, allele, and estimated haplotype frequencies of the patients and comparison group were compared.
At the third stage, patients with a possible hemizygote at the COMT locus were subjected to microdeletion detection at the region critical to velocardiofacial syndrome by using five polymorphic markers, i.e., D22S420, D22S941, D22S947, D22S264, and D22S311, as described elsewhere
(7).
Comparisons of the genotype, allele, and haplotype frequency distributions of the schizophrenic patients and comparison subjects were performed by using chi-square tests. Multisite haplotype frequencies were estimated by using a computer program for population genetics, Arlequin
(8).
RESULTS
Five molecular variants were identified, namely, c.186C>T at exon 3, c.408C>G and c.472G>A at exon 4, c.597G>A at exon 5, and c.821-827insC at the 3′ untranslated region. Only c.472G>A alters an amino acid, from valine to methionine at position 158, while the other mutations are silent mutations. No mutations were identified at exons 1 and 2. Four molecular variants, i.e., c.186C>T, c.472G>A, c.597G>A, and c.821-827insC at the 3′ untranslated region, alter restriction recognition sites, i.e., PmlI, NlaIII, MspI, and BglI, respectively.
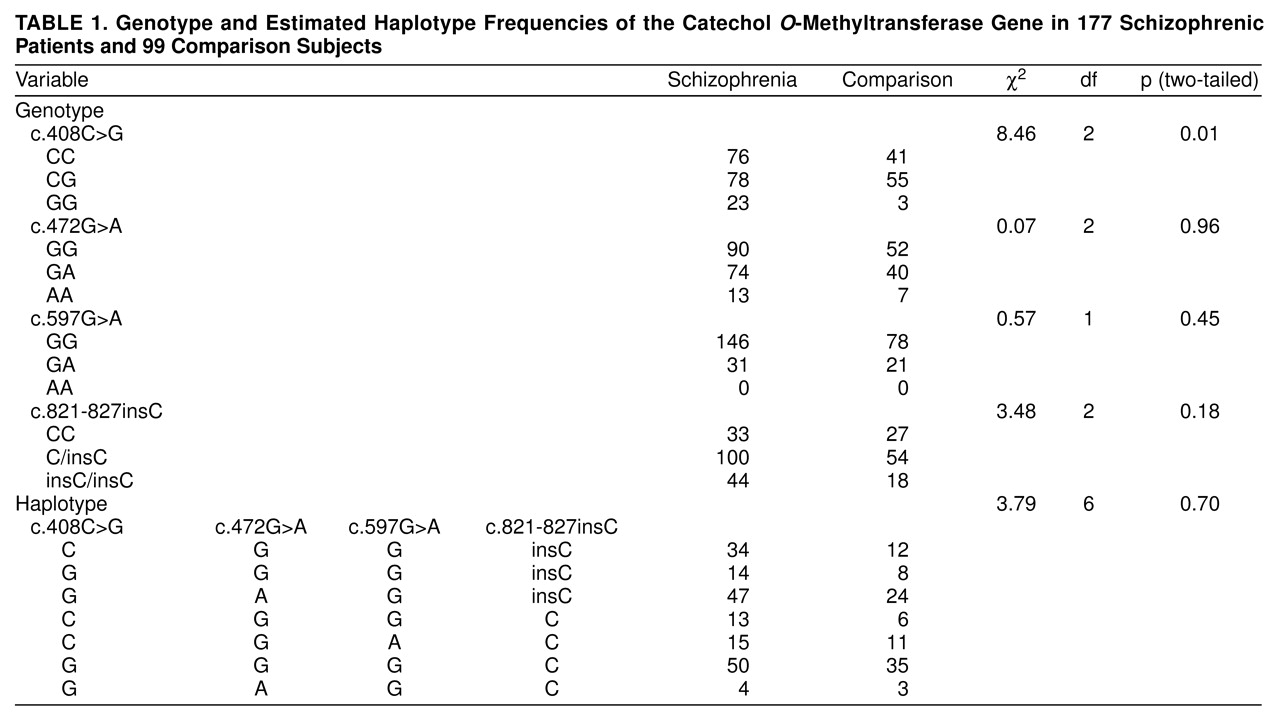
Genetic associations were studied by using the four polymorphisms c.408C>G, c.472G>A, c.597G>A, and c.821-827insC. The genotype and estimated haplotype frequencies are listed in
Table 1. No difference in genotype, allele, or haplotype frequency between the patients and comparison subjects was detected, except for a marginally significant difference of the genotype distribution of c.408C> G (
Table 1).
Furthermore, 28 patients who were homozygous for the four polymorphic markers of the COMT gene were heterozygous for the five polymorphic markers scanning at chromosome 22q11.
DISCUSSION
In this study we identified five polymorphisms of the COMT gene. Only c.472G>A alters an amino acid, from valine to methionine at codon 158; the other four molecular variations are silent mutations. The G allele encoding a valine has three- to fourfold higher COMT activity than the A allele encoding a methionine
(9). The c.472G>A polymorphism is the only functional variant of the COMT gene, to our knowledge, reported in the literature, and it was present in our study group. Further study of c.472G>A, however, did not show an association with schizophrenia in our patients, which is consistent with findings from other studies
(10,
11). Nevertheless, several groups of investigators have reported that the c.472G>A variant is associated with aggression in schizophrenia and schizoaffective disorder
(12), polysubstance abuse
(13), and obsessive-compulsive disorder
(14). Hence, the c.472G>A variant of the COMT gene may contribute to certain behavior traits in mental disorders but not to schizophrenia itself.
Only the c.408C>G polymorphism was found to have a marginally significant difference in genotypic distribution between the patients and comparison group (
Table 1). The p value (0.01) should be corrected for multiple tests. Further haplotype analysis did not reveal significant differences between the patients and comparison subjects. Hence, we do not think the association of the c.408C>G polymorphism is a true positive finding.
In our patients no hemizygote was identified at the region critical to velocardiofacial syndrome. This finding is different from that of other research groups
(15). The discrepancy can be attributed to different methods in recruiting index patients. Our patients were unscreened schizophrenic patients, whereas the patients in other groups were screened first to meet the physical characteristics of velocardiofacial syndrome. Our results indicate that the 22q11 deletion syndrome is rare in our schizophrenic patients.
In summary, our data suggest that the COMT gene is unlikely to play a major role in the etiology of schizophrenia. It would be worthwhile to look for mutations of the other candidate genes at chromosome 22q12.