Twin studies of bipolar disorder have frequently shown a higher concordance for the disease in monozygotic than in dizygotic twins, indicating the importance of a genetic contribution to the liability to this disorder
(1). Probandwise concordance rates have varied from 0.33 to 0.75 for monozygotic twins and from zero to 0.13 for dizygotic twins. Disparities in diagnostic criteria, ascertainment method, or sample compilation among studies can explain this large variability. The few representative studies distinguishing bipolar I disorder from unipolar depressive disorder have all had some methodological limitations
(2–
4). The role of environmental risk factors, such as labor and delivery complications at birth, has been controversial
(5,
6).
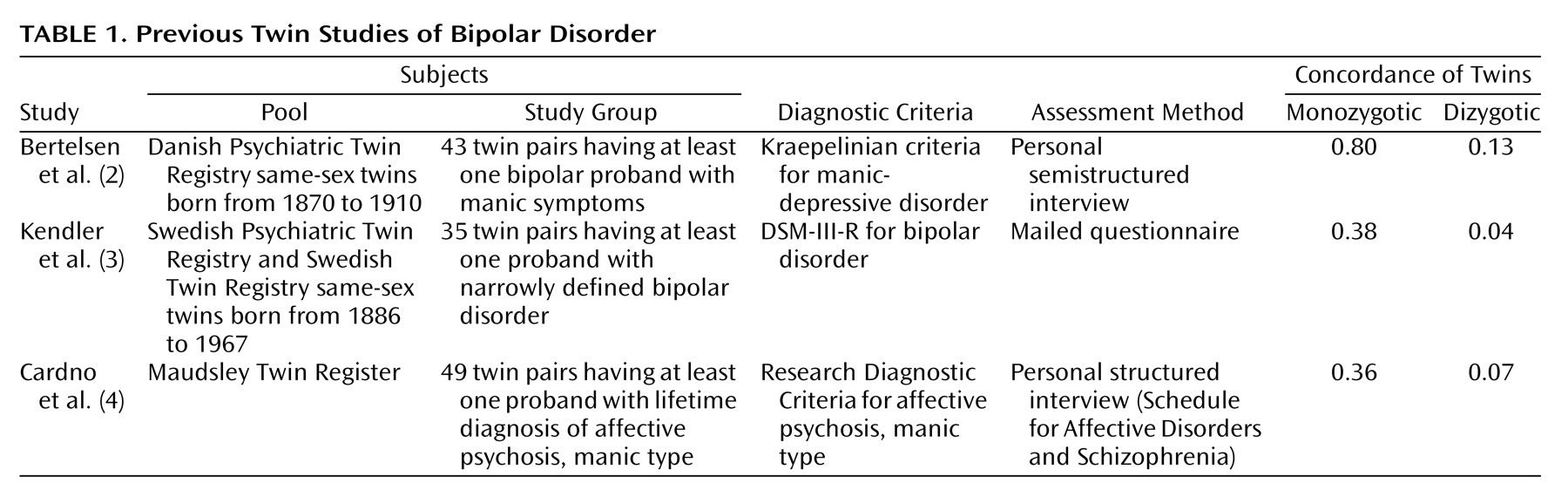
Previous twin studies are briefly characterized in
Table 1. In the Danish study
(2), no structured interview schedule was used, and the diagnoses were made according to Kraepelin’s concept, which is less definitive than that in current diagnostic systems. In the Swedish study
(3), diagnoses were based on self-assessment through a mailed questionnaire, to which the overall response rate was low. Cardno et al.
(4) concentrated on the full range of nonorganic psychoses, and they did not actually diagnose bipolar I disorder but, instead, assessed the lifetime occurrence of affective psychosis, manic type, according to the Research Diagnostic Criteria
(7). In another study of the same subjects
(8), they used the DSM-IV criteria but combined patients with bipolar I and bipolar II disorder. They reported concordances of 0.40 for monozygotic twins and 0.05 for dizygotic twins and a heritability estimate of 0.85.
Our study involved a representative nationwide twin sample with bipolar disorder diagnosed by using a structured method with face-to-face interviews. Here we report the concordance rates, correlations in liability, and estimates of heritability from biometrical model fitting. Heritability is the proportion of variation of a feature in the population that is accounted for by genetic factors
(9). A correlation in liability refers to the extent to which the liability (a whole combination of external and internal circumstances that makes one more or less likely to develop the disease) of a twin predicts the liability of a co-twin
(10). We also investigated possible environmental factors, such as prenatal, obstetric, and early childhood complications, that might explain discordance between the twins.
Results
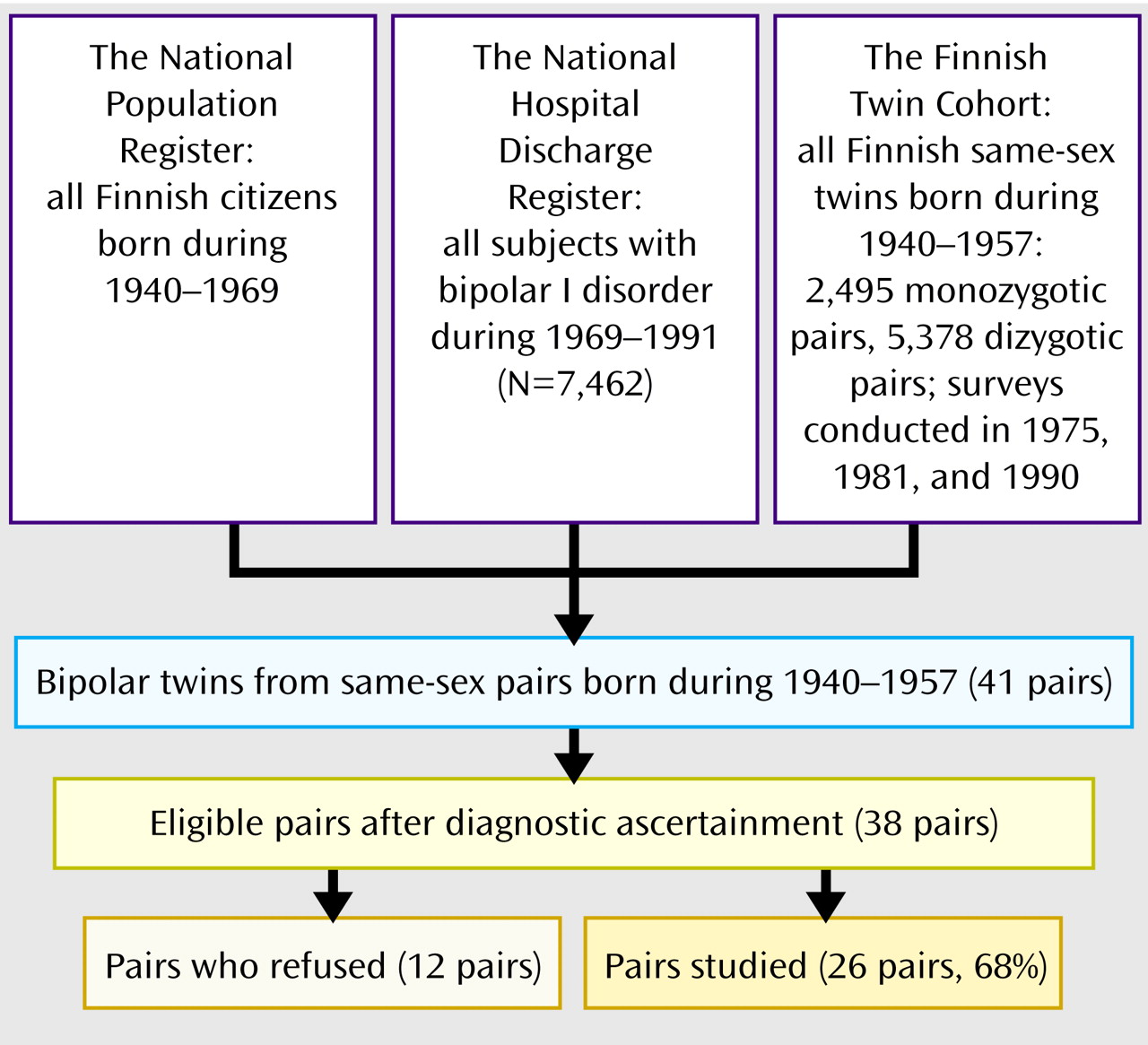
Subject Characteristics
Of the 27 probands, 25 had bipolar I disorder and two had schizoaffective disorder, bipolar type, as assessed with the SCID
(16). Seven of the 26 pairs were monozygotic, and 19 were dizygotic, a distribution that accords with the overall numbers of 2,495 confirmed monozygotic and 5,378 confirmed dizygotic twin pairs (χ
2=0.27, df=1, p=0.60). The mean age of the twins at the end of follow-up was 48 years (SD=5, range=37–57).
Incidence
The number of new cases of bipolar I disorder during the follow-up was 22, and the number of person-years derived from the Finnish Twin Cohort was 290,028. The overall annual incidence of bipolar I disorder per 100,000 population was 7.6, with a 95% confidence interval (CI) of 4.4 to 10.8. The rate was 6.9 for women (95% CI=2.6 to 11.2) and 8.3 for men (95% CI=3.6 to 13.0). Using the same registers, we also estimated the incidence of bipolar I disorder in the whole Finnish population. During the follow-up period of 1970–1991, the annual incidence in the 1954–1959 birth cohort was 5.8 (95% CI=5.4 to 6.3).
The number of bipolar I patients ascertained from medical records was 38, while the number of all twins at the beginning of the follow-up was 19,124. This yielded the cumulative incidence (morbid risk) of 0.2%.
Testing for Biases
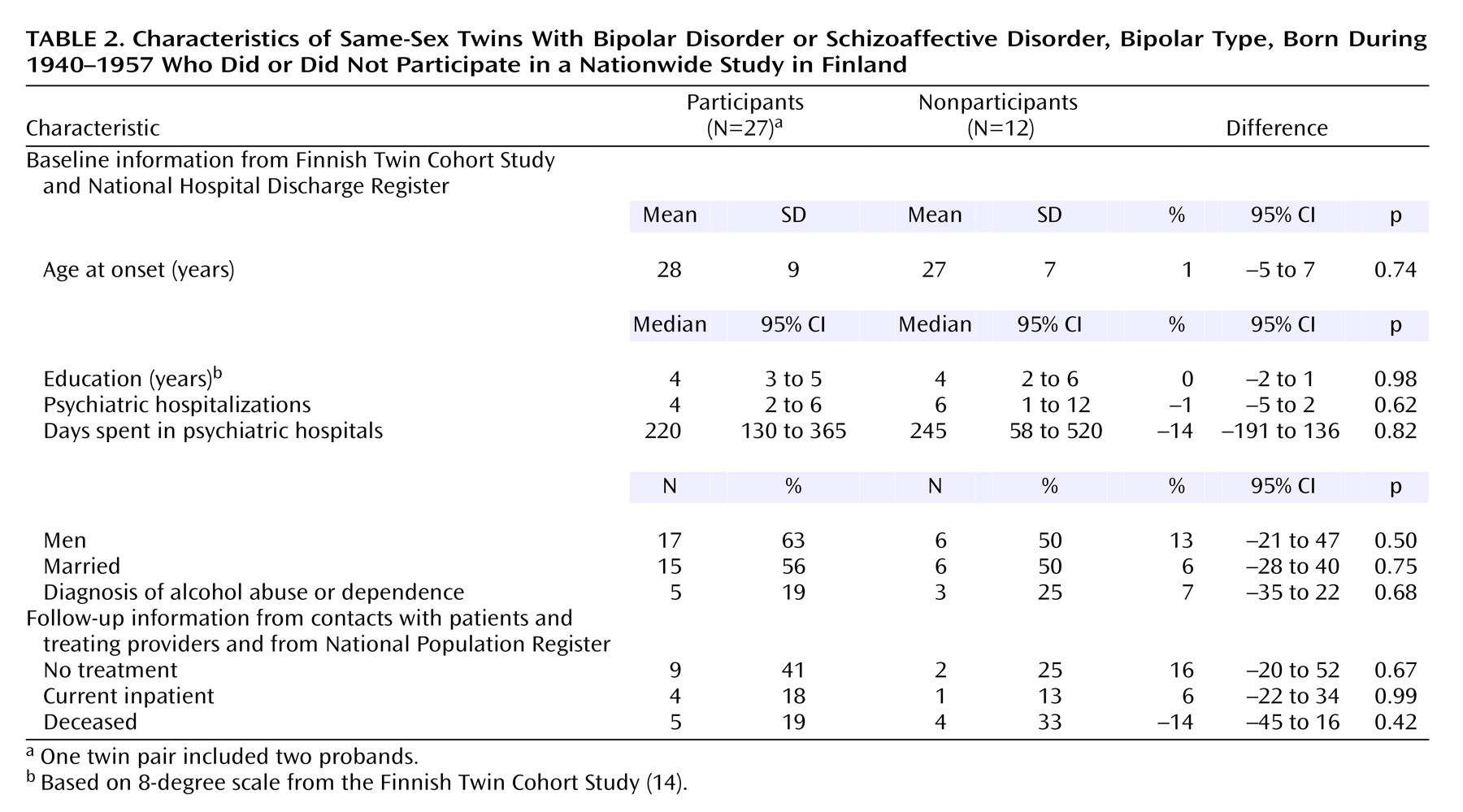
No significant differences were observed between the participants and nonparticipants (
Table 2). The mean length of cohabitation was 3 years longer (z=–2.57, p=0.01) among the monozygotic than the dizygotic twins, and the frequency of contacts in adulthood was higher (z=–2.95, p=0.003). However, no association was found between affection status and either the length of cohabitation (N=38, p=0.66) or the degree of environmental sharing (N=50, p=0.17). None of the possible confounding factors (type of diagnostic ascertainment, type of zygosity determination, sex, education, diagnosis of alcohol abuse or dependence, premorbid organic pathology, or age) showed a significant association (all p>0.18) with the status of the co-twin.
Concordance and Correlations in Liability
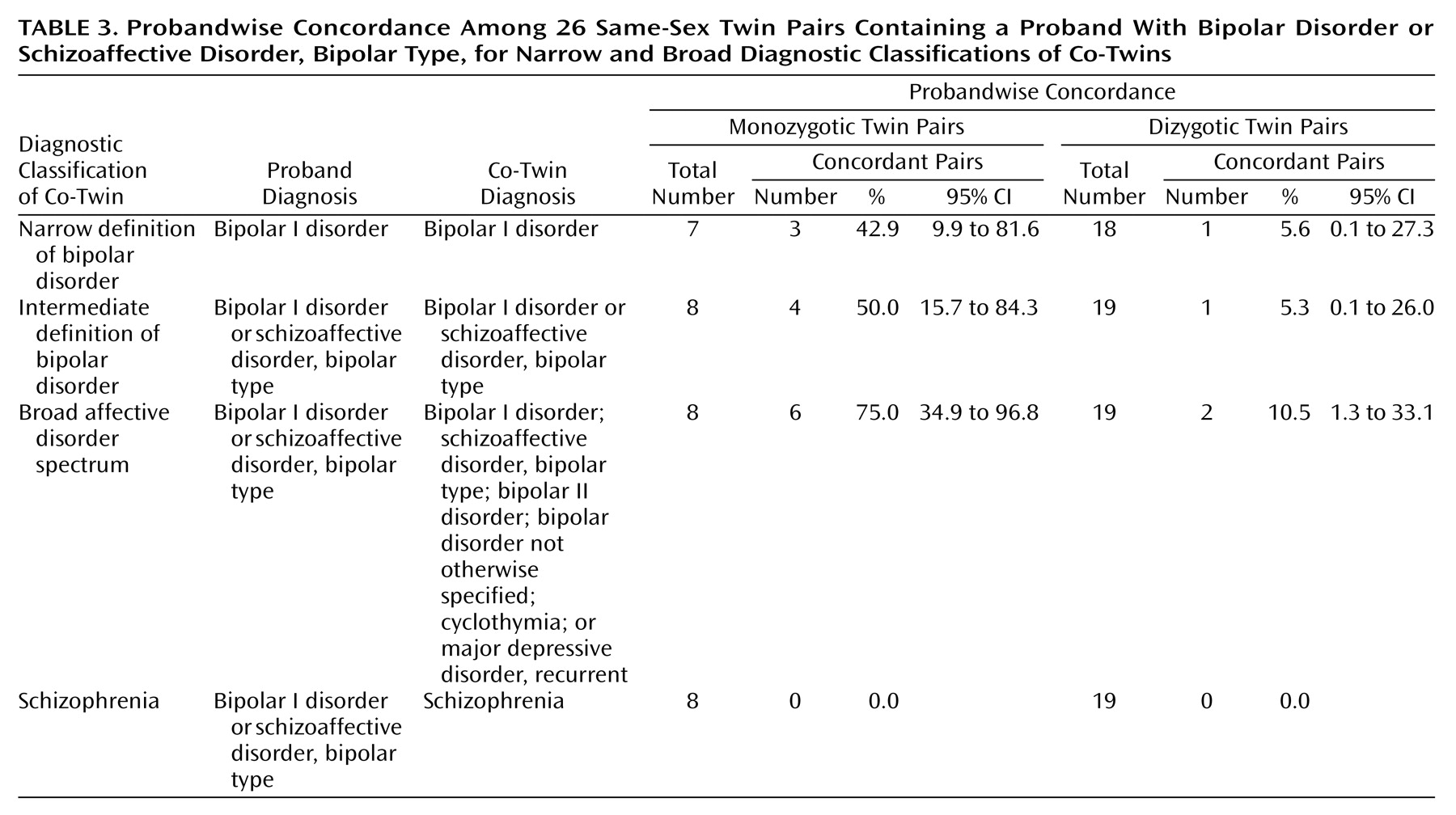
The probandwise concordance rates for different diagnostic classifications related to bipolar disorder are shown in
Table 3. The concordance for bipolar I disorder was 0.43 for monozygotic twins and 0.06 for dizygotic twins. When we included patients with schizoaffective disorder, manic type, the rates were 0.50 and 0.05, respectively. The concordance for the broad affective disorder spectrum was 0.75 for monozygotic twins and 0.11 for dizygotic twins. No cases of schizophrenia occurred in this sample. The correlations in liability for bipolar I disorder were 0.85 (95% CI=0.28 to 0.98) for monozygotic twins and 0.41 (95% CI=0.00 to 0.73) for dizygotic twins. Although the concordance rates and correlations in liability for bipolar I disorder were greater for monozygotic twins than for dizygotic twins, the differences were not significant.
We recalculated the concordance rates by using the pairs for which zygosity was based on genetic marker analysis (18 of 26 pairs). The probandwise concordance rates for bipolar I disorder were 0.33 (two of six) for monozygotic twins and 0.08 (one of 13) for dizygotic twins, and the concordance rates for bipolar I disorder plus schizoaffective disorder, bipolar type, were 0.43 (three of seven pairs) and 0.07 (one of 14), respectively. The rates did not differ from the rates derived from the whole sample (Fisher’s exact test).
We also calculated pairwise concordance rates for the same diagnostic categories. The pairwise concordance for bipolar I disorder was 0.33 for monozygotic twins and 0.06 for dizygotic twins (p=0.15). When we included patients with schizoaffective disorder, manic type, the rates were 0.43 and 0.05, respectively (p=0.05, Fisher’s exact test), and the concordance for the broad affective spectrum was 0.71 for monozygotic twins and 0.10 for dizygotic twins (p=0.006, Fisher’s exact test).
Model Fitting
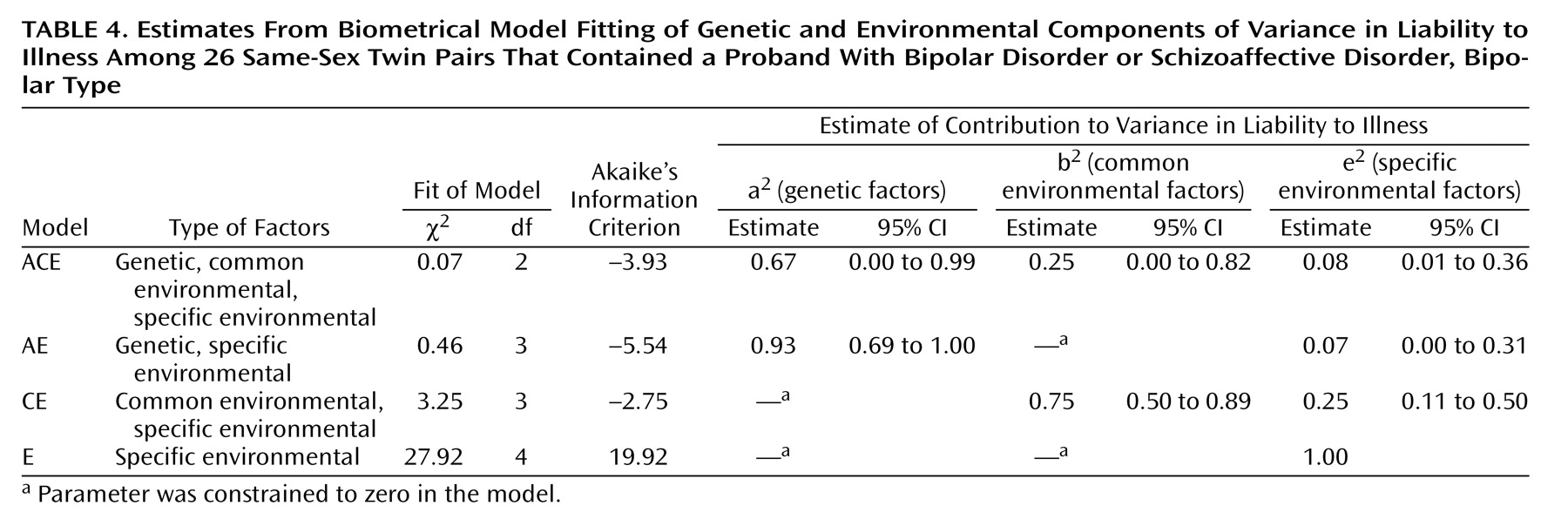
Table 4 shows the results of biometrical model fitting for bipolar I disorder. The model with only specific environmental factors explaining the variance in liability (E) was rejected by the chi-square test. The model with common and specific environmental components explaining the variance (CE) could not be rejected, but it fitted clearly worse than the models involving genetic effects (ACE and AE). On the grounds of parsimony, the model of genetic and specific environmental factors (AE) was the best-fitting model, with a heritability estimate of 0.93. However, it should be noted that in the full model (ACE), the confidence limits for both genetic and environmental factors included zero.
Environmental Risk Factors
Reports from maternity clinics were available for 80.8% of the mothers. There were no significant differences between the concordant and discordant pairs in physical or mental problems during pregnancy or delivery (Fisher’s exact test). Birth clinic information was available for 96.2% of the twins. There were no significant differences in reported postnatal complications between the probands with bipolar I disorder and the co-twins (F=0.04, df=1, 28, p=0.85). The mean heights were 47.3 cm and 47.4 cm (F=0.14, df=1, 20, p=0.71), and the mean weights were 2503 g and 2698 g (F=2.60, df=1, 26, p=0.12), respectively. Reports from child welfare clinics were available for 67.3% of the study subjects. The bipolar probands and healthy co-twins did not differ from each other in the occurrence of childhood infections (F=0.44, df=1, 23, p=0.51) or reported physical or behavioral complications (F=1.56, df=1, 22, p=0.24).
Discussion
To our knowledge, this is the first study that has evaluated the concordance rates and heritability for bipolar I disorder in a representative and well-defined twin sample with modern diagnostic assessment through personal interviews. Our concordance rates are almost identical to those of two studies
(3,
4) employing standardized diagnostic systems, suggesting a considerable between-population stability of the genetic contribution to the liability for bipolar disorder. Although there are limitations from the genetic viewpoint in the use of concordance rates
(22), they offer a simple way to compare results in different studies, provided that the prevalence of disease does not differ between them. The Danish study
(2) produced higher concordance rates for both monozygotic and dizygotic twins. The semistructured diagnostic method used could partly explain the higher concordance, although the longer follow-up period could also be a factor.
The inclusion of schizoaffective disorder, bipolar type (two pairs), did not change the concordance rate much from the rate for pure bipolar I disorder. This similarity accords with the assumption that schizoaffective disorder, bipolar type, and bipolar I disorder have a common genetic background
(15,
23). The phenotype might more precisely be a manic behavior (a manic polarity of the affective continuum). The findings of Cardno et al.
(4) support the hypothesis of an affective status (especially a manic state) as the key heritable trait. Their reported concordance rates for the lifetime occurrence of mania were close to our rates for bipolar I disorder. In our study there were no cases of schizophrenia among the co-twins, a finding that somewhat strengthens the concept that schizophrenia and bipolar I disorder are distinct disorders.
When evaluating the concordance rates for different definitions of the disorder, we found, like Bertelsen et al.
(2), that they were higher for the broad affective spectrum, especially in monozygotic twins. It is possible that monozygotic co-twins with bipolar II disorder or unipolar disorder, who were included in this category, could have a genotype for bipolar I disorder but only a partial phenotype that will later develop into the full disorder. Although the nature of the relationship between bipolar I disorder and bipolar II disorder is controversial, follow-up studies indicate that 4%–7% of patients with bipolar II disorder develop full mania in 3 to 10 years
(24,
25). Of patients with unipolar disorder, 12%–41% eventually develop mania or hypomania
(26,
27). The genetic relationship between bipolar I disorder and unipolar disorder has received special interest over the past three decades. The literature offers three models of transmission of bipolar I disorder and unipolar disorder
(28): 1) a model in which they are nearly independent
(29), 2) a multiple-threshold model in which bipolar I disorder represents a more severe form of the same familial condition
(8,
28,
30), and 3) a model that does not subtype bipolar I disorder and unipolar disorder according to familiality but treats them as differentiated mainly by nonfamilial factors
(31). Our results are in agreement with both the second and third models.
The correlations in liability to bipolar I disorder were much greater for monozygotic than dizygotic twins, indicating the importance of the genetic contribution. As expected, they closely resembled the findings of Kendler et al.
(28) (0.80 for monozygotic, 0.28 for dizygotic) and Cardno et al.
(4) (0.82 for monozygotic, –0.31 for dizygotic), despite the relatively small sample sizes of all three studies. We were unable to conclusively reject the model with no genetic component, but it fitted clearly worse than the models with both genetic and environmental components. The best-fitting model, the AE model (with the variance explained by common environmental factors constrained to zero), gave a heritability estimate of 0.93 for bipolar I disorder, while the ACE model produced a heritability of 0.67. Both Kendler et al.
(28) and Cardno et al.
(4) reported that the AE model was the best fitting, with heritability estimates of 0.79 and 0.84, respectively. They assumed higher morbid risks (1.6% and 1.5%, respectively) than we did, but Cardno et al.
(4) also applied a 0.1% morbid risk estimate for mania, which then produced a heritability estimate of 0.88. Our estimate agrees with that. We were unable to differentiate between the proportions of additive and dominance genetic effects, and the modeling results must in any case be interpreted with caution.
The sample size of our study was relatively small, which limited the power to reject inappropriate models. The failure to find a statistically significant difference in concordance for bipolar I disorder between monozygotic and dizygotic twins is probably due to a lack of power. Indeed, the addition of two pairs (probands having schizoaffective disorder, bipolar type) gave a p value of 0.05. Six of the twins were deceased, but five of them were probands, and we were able to get carefully collected information about their medical history. Three of them had committed suicide. That is 11% of the study population, a proportion that is well in accordance with suicide rates among bipolar patients in other studies
(32). A dizygotic co-twin had also committed suicide, although he had no previous psychiatric history according to records and interviews with relatives. Thus, it seems unlikely that he had had bipolar I disorder.
Although the sample was small, it represented the total population, being derived from the National Population Register and the Finnish Twin Cohort. The probands were screened by using data from the twin cohort surveys and the National Hospital Discharge Register for the follow-up period of 1969 to 1991. The annual incidence of bipolar I disorder in the sample was in accordance with rates in previous studies
(33,
34). We were able to check the annual incidence in the Finnish 1954–1959 birth cohort during the follow-up period 1970–1991 and found it to be well in accordance with the incidence in our twin population.
The heritability estimate may be biased if there is substantial assortative mating for the disease. Another assumption underlying the model fitting is the multifactorial threshold model, which presupposes many common genes with modest effect sizes in the population
(35). However, most of the evidence to date supports this assumption
(36). It is noteworthy that twins classified concordant for nonaffection (according to the National Hospital Discharge Register and the Finnish Twin Cohort surveys) were not interviewed. The number of discordant pairs may thus have been underestimated and caused overestimation of familiality. However, there is no reason to believe that the underestimation would differ by zygosity, and the incidence of disease corresponded to expectation. Likewise, our period of primary ascertainment was not the twin’s lifetime but the several decades for which register information was available, yet all twins were interviewed for lifetime history. Again, this effect would be unlikely to depend on zygosity. Thus, our estimate of familiality may be somewhat biased upward.
Environmental factors, including measurement error, accounted for 7%–33% of the variance in liability. In addition to chance and errors, these could involve obstetric complications
(5), infections during pregnancy or early childhood
(37), and early losses
(38). Our study did not give support to the hypothesis that complications during pregnancy, at birth, or postnatally or early childhood infections could explain vulnerability to bipolar I disorder. Preschool physical or behavioral complications were not more common among the probands than among the co-twins.
We believe that this is the first twin study of bipolar disorder involving a representative nationwide twin sample in which bipolar I disorder was diagnosed by using structured face-to-face interviews and long-term follow-up data. Our results confirm previous findings that the heritability for bipolar I disorder is high. However, in the future we need greater insight into the most heritable traits or components of bipolar I disorder and the specific environmental factors that could either increase the risk of its development or prevent it.