Seasonal affective disorder is the term applied to a clinical subtype of mood disorder that consists of recurrent episodes of major depression occurring with a seasonal pattern
(1) . The most common type of seasonal affective disorder is winter depression in which patients experience symptoms of clinical depression during the fall and winter, with full remission to normal mood (or a switch into mania or hypomania) during the spring and summer seasons. Symptoms of seasonal affective disorder include depressed mood, profound lack of energy, hypersomnia, hyperphagia with carbohydrate craving, and weight gain
(2) . Seasonal affective disorder is also associated with significant impairment in occupational and social functioning
(3,
4) . Epidemiological studies from Canada and the United States, using diagnostic interviews conducted among random community samples, have reported winter seasonal affective disorder rates of between 0.4% and 2.7% in the general population
(5 –
7) . There is considerable evidence that seasonal affective disorder is effectively treated by daily exposure to bright artificial light, known as light therapy or phototherapy, and by antidepressant medication.
More than 70 controlled studies of light therapy for seasonal affective disorder have been conducted. An early pooled analysis of over two dozen studies found that bright light treatment was superior to control conditions (usually dim light) but primarily in less severely ill patients
(8) . However, these findings were criticized for methodological limitations, including small sample sizes and short treatment durations (1–2 weeks) of the included studies. Subsequently, two randomized controlled trials with larger sample sizes and longer durations found that bright light therapy using fluorescent light boxes was superior to plausible placebo control conditions
(9,
10) . Terman et al.
(9) studied 144 patients with seasonal affective disorder randomly assigned to one of four treatments for 2–4 weeks: 1) morning or 2) evening bright light (exposure to 10,000-lux fluorescent light for 30 minutes) or 3) high-density or 4) low-density negative ions emitted from a negative ion generator (the placebo condition). Eastman and colleagues
(10) studied 96 patients randomly assigned to 5 weeks of treatment with morning or evening bright light (consisting of a 6,000-lux fluorescent light box for 1.5 hours), or morning use of a deactivated negative ion generator (the placebo condition). In both studies, bright light was superior to the placebo condition in producing clinical remissions, and morning light exposure was superior to evening on some measures. In addition, three systematic reviews incorporating meta-analyses have also supported the efficacy of light therapy, although it was noted that the treatment duration of included studies remained relatively short (5 weeks or less)
(11 –
13) . This evidence resulted in the recommendation of light therapy as a first-line treatment for seasonal affective disorder in expert and consensus clinical guidelines
(14 –
17) .
Antidepressant medications have not been studied as extensively as light therapy in the treatment of seasonal affective disorder. Selective serotonin reuptake inhibitors (SSRIs) have the best-demonstrated evidence for medication efficacy. In one study, patients with seasonal affective disorder (N=68) were randomly assigned to treatment with fluoxetine, 20 mg/day, or placebo for 5 weeks
(18) . The improvement in overall depression scores was not significantly different, but the effect size of 0.5 for fluoxetine was similar to that found in other antidepressant trials for nonseasonal depression. In addition, the clinical response rate (greater than 50% improvement in depression scores) for fluoxetine was significantly higher than placebo (59% versus 34%, respectively). In the subset of patients who were more severely ill at baseline, fluoxetine did show statistical superiority in improving the overall depression scores. In a larger study (N=187), sertraline in a flexible dose (50–200 mg/day) for 8 weeks was superior to placebo both in improving depression scores and in the clinical response rate (63% versus 46%, respectively)
(19) . In both studies, the SSRI drugs were well tolerated, with few dropouts in any condition (between 3.1% and 7.5%).
Smaller controlled studies have shown that other medications, including moclobemide,
l -tryptophan, and hypericum (St. John’s wort), may be effective treatments for seasonal affective disorder. There have also been case series suggesting that bupropion, citalopram, reboxetine, and tranylcypromine are beneficial (reviewed by Lam and Levitt
[15] ).
In summary, both light therapy and antidepressants have evidence showing efficacy in seasonal affective disorder treatment and are considered first-line therapies. An important clinical question remains: How does light therapy compare with antidepressant treatment? There are few systematic comparisons of light therapy versus antidepressant drugs in seasonal affective disorder. A single-case study suggested that citalopram, an SSRI antidepressant, was as effective as light therapy
(20) . A small randomized controlled trial (N=35) compared bright light therapy (3,000 lux, 2 hours/day) combined with placebo capsules versus fluoxetine (20 mg/day) combined with placebo light (100 lux, 2 hours/day) for 5 weeks in patients with seasonal affective disorder
(21) . Both conditions produced significant response, with no difference in final depression scores and no difference in clinical response rates (>50% reduction in depression scores: light therapy, 70%; fluoxetine, 65%). However, when strict criteria for clinical remission were used (>50% reduction in depression scores
and a posttreatment score within the normal range), light therapy showed a superiority over fluoxetine (50% versus 25%) that approached significance (p=0.10). In addition, post hoc testing showed that light therapy resulted in significantly lower depression scores after 1 week of treatment. There were no differences between treatments at other time points. The limitations of this study were its small size (hence low power to detect differences) and that the timing of exposure to light (morning, evening, or morning and evening) was chosen by the patient. In fact, there was some suggestion that morning light was more effective, since 10 of 12 patients responded to morning light, compared with two of five for evening light, and two of three for morning and evening light. Using the more optimal morning timing of light exposure for all patients may have further added to the superior response rate of light therapy over fluoxetine.
Important questions that remain to be answered for the clinical treatment of seasonal affective disorder are 1) whether light therapy is effective over longer treatment periods, since controlled treatment studies have only been 1–5 weeks in duration, and 2) how light therapy compares with antidepressant drugs, especially for more severely ill patients. To help answer these questions, we conducted a multicenter randomized controlled trial that compared the effectiveness of light therapy to the SSRI antidepressant fluoxetine. We randomly assigned depressed patients with seasonal affective disorder recruited from four Canadian cities to 8 weeks of treatment during the winter. To balance potential expectation effects, each patient received both a light box and a pill, but only one treatment was active in each condition.
Method
Protocol
This randomized, double-blind study was approved by a clinical research ethics board at each center. After giving written informed consent, eligible subjects entered a 1-week baseline phase without treatment to regularize their sleep-wake schedule (patients were instructed to sleep only between the hours of 10:00 p.m. and 8:00 a.m.) and to identify spontaneous responders. Patients who were significantly improved after the baseline week (defined as 25% or greater improvement in depression scores) were dropped from the study. Otherwise, they were randomly allocated to one of two treatment conditions for 8 weeks: active light therapy plus placebo capsules, or placebo light therapy plus active drug. Randomization codes were centrally computer generated and stratified by site in random blocks of 3–5. Allocation concealment used opaque envelopes at each site that could only be opened after the unique subject number was entered in a master log. Patients returned to the clinic for outcome assessments at weeks 1, 2, 4, and 8 or at unexpected termination.
Subjects
Subjects were recruited by referral and advertisements at mood disorders clinics in 1) Vancouver, 2) Winnipeg, 3) Toronto, and 4) Saint John, New Brunswick. The inclusion criteria for the study were male and female outpatients 18–65 years of age who had major depressive episodes with a seasonal (winter) pattern as determined by the Structured Clinical Interview for DSM-IV (SCID) modified to include criteria for seasonal pattern
(5) . In addition, subjects were required to have a score of 20 or higher on the 17-item Hamilton Depression Rating Scale or a score of 14 or higher on the 17-item version if the score on a 24-item version (subsequently described) was 23 or higher. Patients had to meet these criteria, which indicate moderate to severe depression, both at initial assessment and at the end of the baseline week.
Subjects were excluded from the study if they 1) were pregnant or lactating (or were sexually active women of childbearing potential not using medically accepted means of contraception); 2) were at serious suicidal risk in the judgment of the investigator; 3) met DSM-IV criteria for organic mental disorders, substance use disorders (including alcohol) within the last year, schizophrenia, paranoid or delusional disorders, other psychotic disorders, bipolar I disorder, panic disorder, or generalized anxiety disorder not concurrent with major depressive episodes; 4) had a serious unstable medical illness; 5) had retinal disease that precluded the use of bright light; 6) had a history of severe allergies or multiple drug adverse reactions; 7) were currently using other psychotropic drugs including lithium, l -tryptophan, St. John’s wort, or melatonin; 8) were currently using beta blocking drugs; 9) had used antidepressants or mood-altering medications within 7 days of baseline; 10) had been treated previously with fluoxetine or light therapy; 11) had undergone formal psychotherapy (e.g., cognitive behavior or interpersonal psychotherapy) in the 3 months preceding the study or initiated it during the study itself; or 12) performed shift work or traveled south during the protocol.
Subjects were entered into the study during the autumn and winter months starting from Sept. 15. Enrollment was stopped by Feb. 15 in order to reduce the possibility of spontaneous spring remission. The study was conducted over three winter seasons (2000/2001–2002/2003).
Light Treatment
The active light treatment consisted of daily exposure to a white fluorescent light box (Uplift Technologies Inc. [Dartmouth, N.S.], Model Daylight 10000, fitted with an ultraviolet filter and rated at 10,000 lux at a distance of 14 in from screen to cornea) for 30 minutes as soon as possible after awakening, between 7:00 a.m. and 8:00 a.m. A suitable placebo condition for bright light is problematic and controversial
(23) . In this study, the placebo light treatment was an identical light box fitted with a neutral density gel filter to reduce light exposure to 100 lux. Deception was used to enhance the plausibility of the light control condition by explaining to patients (using a structured script) that the objective of the study was to examine different wavelengths of light and light boxes, without mentioning the different intensities. After being shown the assigned light box, pretreatment ratings of expectations for light therapy (and separately for medication) were measured with a modified Expectation of Response questionnaire
(24) used in other seasonal affective disorder studies
(25) . On study completion, the patients were debriefed and allowed to continue receiving active light treatment if they wished.
Patients were given verbal and written instructions on the use of the light box and a measurement tape was used to ensure proper positioning. Illumination intensities were confirmed by digital photometer. Adherence was measured by using daily logs of treatment times that were completed by subjects and reviewed at each visit. Patients were also instructed to avoid spending an excessive or unusual amount of time outdoors during the entire study period.
Medication Treatment
The active medication treatment was a daily, fixed dose of fluoxetine, 20 mg/day, taken between 7:00 a.m. and 8:00 a.m. while the placebo was an identical capsule containing inert filler. Adherence was measured by pill counts at each visit. In addition, blood samples were taken at the completion of the study, and a random subset of samples were assayed for serum fluoxetine levels.
Outcome Measures
The primary outcome measure was the 24-item Hamilton depression scale score obtained by board-certified psychiatrists blind to treatment assignment (the blind was maintained by having a separate research assistant managing the light device treatment and asking patients not to discuss side effects or specifics of treatment with the rater). A semistructured interview, the Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (SIGH-SAD)
(22) was used to increase reliability. The SIGH-SAD generates scores for several versions of the Hamilton depression scale, including the 17-item version, the 21-item version, and an eight-item atypical symptom addendum. This atypical addendum was included because the original Hamilton scale does not rate symptoms such as hypersomnia, increased appetite, and weight gain, which predominate in seasonal affective disorder. Some seasonal affective disorder studies report the total 29-item SIGH-SAD score, which consists of the 21-item Hamilton scale plus the eight-item atypical symptom addendum. However, the 17-item Hamilton scale is the most widely used measure of depression severity in clinical trials because the 21-item measure includes four items (diurnal variation of mood, paranoid thoughts, obsessive-compulsive symptoms, depersonalization) originally added to subtype the depressive episode, not to measure severity. Similarly, the eight-item atypical symptom addendum includes one comparable subtyping item reflecting diurnal mood variation (afternoon slump). Therefore, like other seasonal affective disorder studies
(10), we used the Hamilton measure that best reflects severity of depression in seasonal affective disorder, namely the 24-item Hamilton depression scale (comprising 17 “typical symptom” items plus seven “atypical symptom” items). Interrater reliability of the SIGH-SAD was assessed using videotaped interviews with an intraclass correlation of 0.95 for the 24-item Hamilton scale.
Clinical response was defined as 50% or greater reduction from baseline in 24-item Hamilton depression scale scores at the last visit, while clinical remission was defined as clinical response plus a score of 8 or less. Other outcome measures included Clinical Global Impression rating and score on the patient-rated Beck Depression Inventory II, which includes items for atypical symptoms. Adverse effects were monitored using the Adverse Events Scale (unpublished scale from the Canadian Network for Mood and Anxiety Treatments available on request). This self-rated scale assesses both frequency and severity (rated as none, mild, moderate or severe) of 32 adverse events (including a category for “other”) and provides a more comprehensive and systematic evaluation of adverse events than is usually conducted in antidepressant clinical trials. A treatment-emergent adverse event was defined as any increase in rating during treatment to a score of moderate or severe.
Statistical Analysis
All patients randomly assigned to a treatment condition were included in the intent-to-treat analysis, with missing data handled using the last observation carried forward method. Sample size was estimated on the basis of a power analysis using endpoint change scores on the main outcome variable. Assuming a standard deviation of 6 points, a study with 45 patients per condition would allow 80% power to detect a mean difference of at least 3.6 points or an effect size of 0.6, regarded as a medium-sized treatment effect in behavioral studies.
All treatment variables remained coded, and the analysts and investigators were blind to variable identity during the primary analysis and interpretation. The continuous outcome scores were analyzed by using repeated measures analysis of variance (ANOVA), with one within-subject factor (time) and two between-subject factors (treatment condition and site). A Greenhouse-Geisser correction was conducted to adjust for degrees of freedom if the Mauchly Test of Sphericity was significant. In this analysis, differences between treatment conditions would show as significant condition-by-time or condition-by-time-by-site interaction effects. This analysis also assumes compound symmetry, which, if violated, may lead to type I errors greater than the nominal values. Post hoc t tests were done to examine differences between conditions on change scores at each visit. Dichotomous variables were tested by using chi square tests with Fisher’s exact tests as appropriate. All analyses were done by using SPSS v.11.
Results
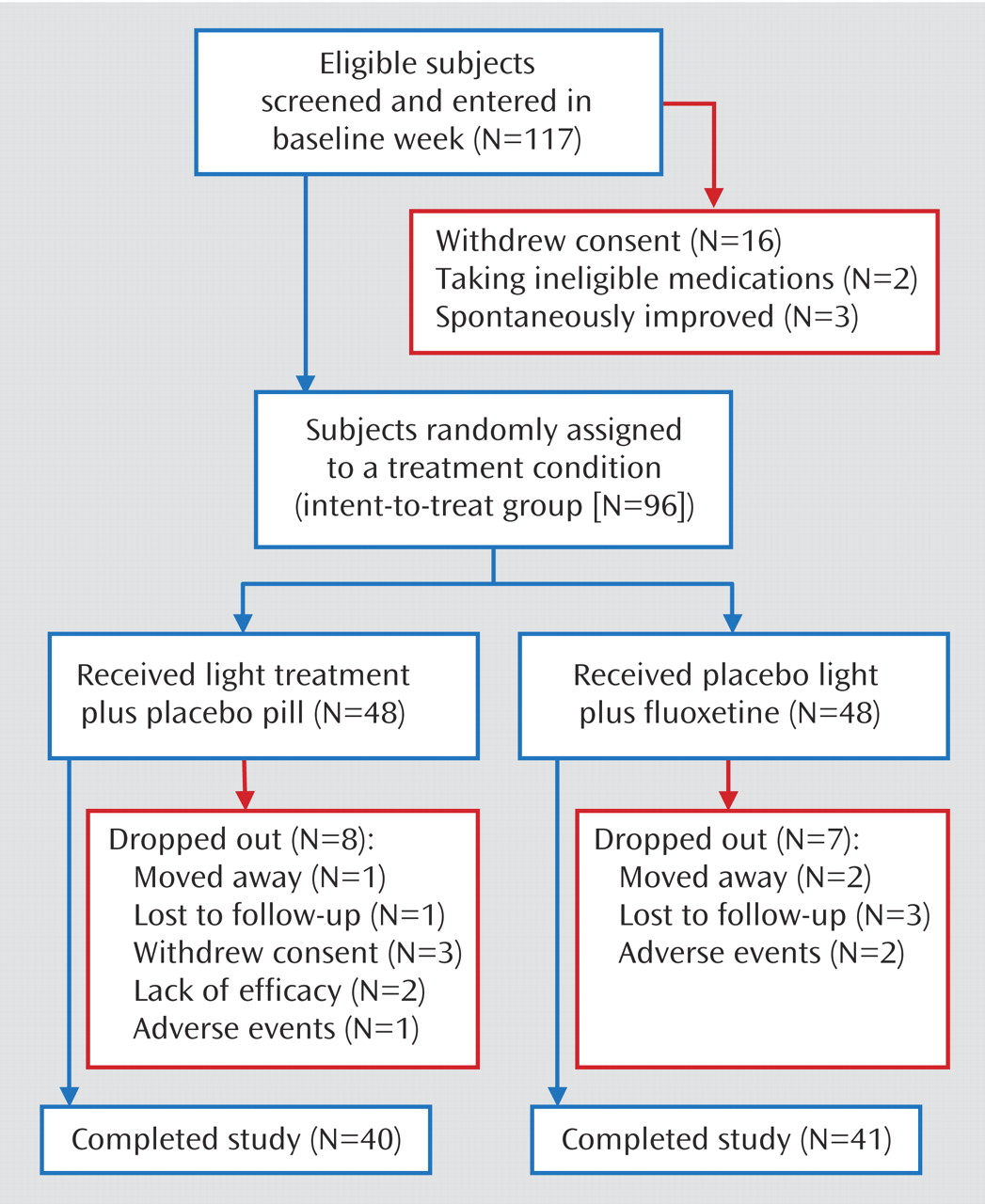
Figure 1 shows the patient numbers through the phases of the study. A total of 96 patients were randomly assigned to a treatment condition.
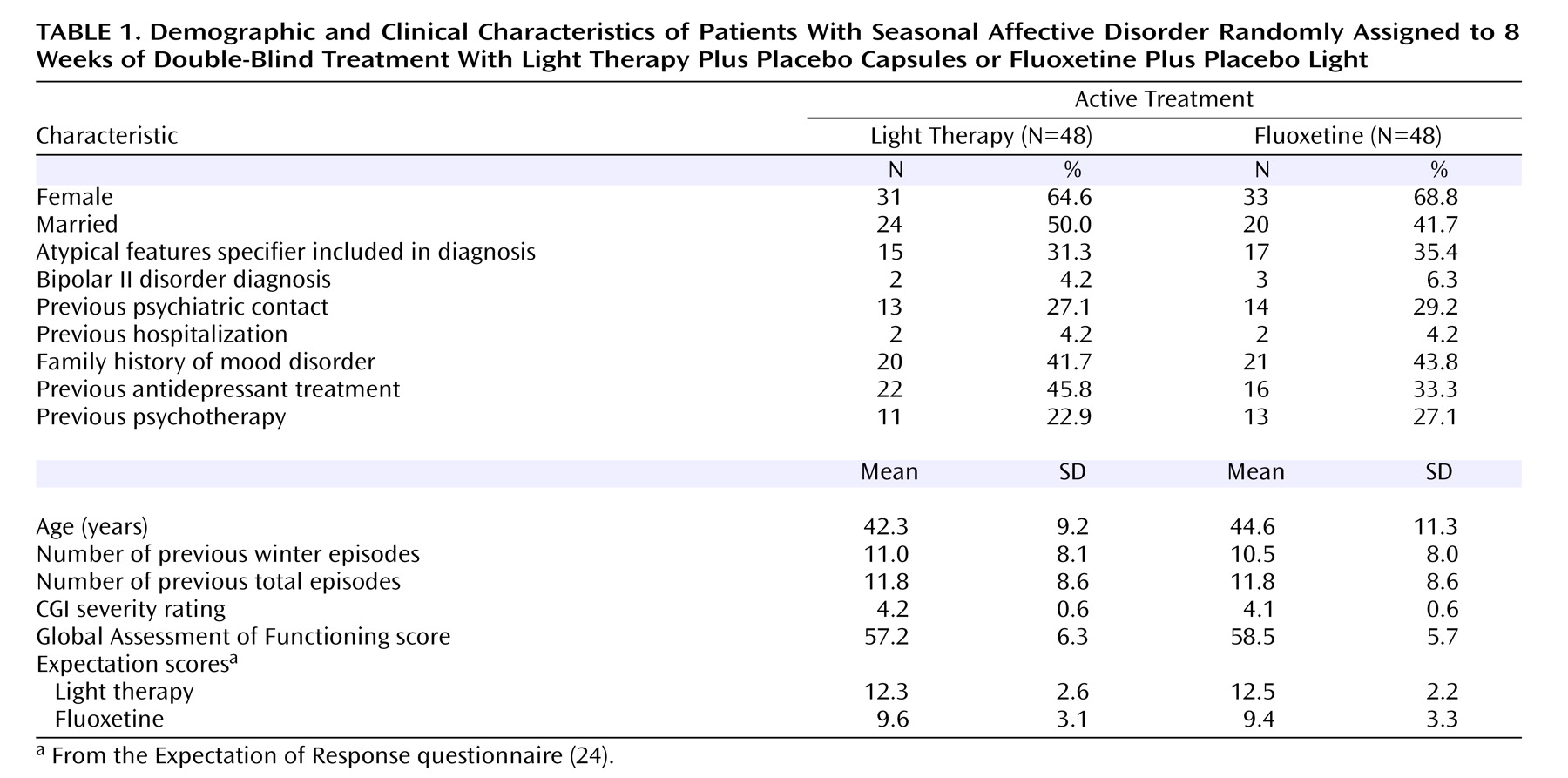
Table 1 shows clinical information on the patients in the two groups. There were no significant differences noted for any of the clinical variables. The analysis of expectation ratings showed a main effect for modality, in that higher expectations were recorded for the light treatment compared with fluoxetine (F=9.8, df=1, 75, p=0.003), but there were no main or interaction effects with treatment condition, so that there were no differences in expectations for light or drug between patients assigned to either treatment.
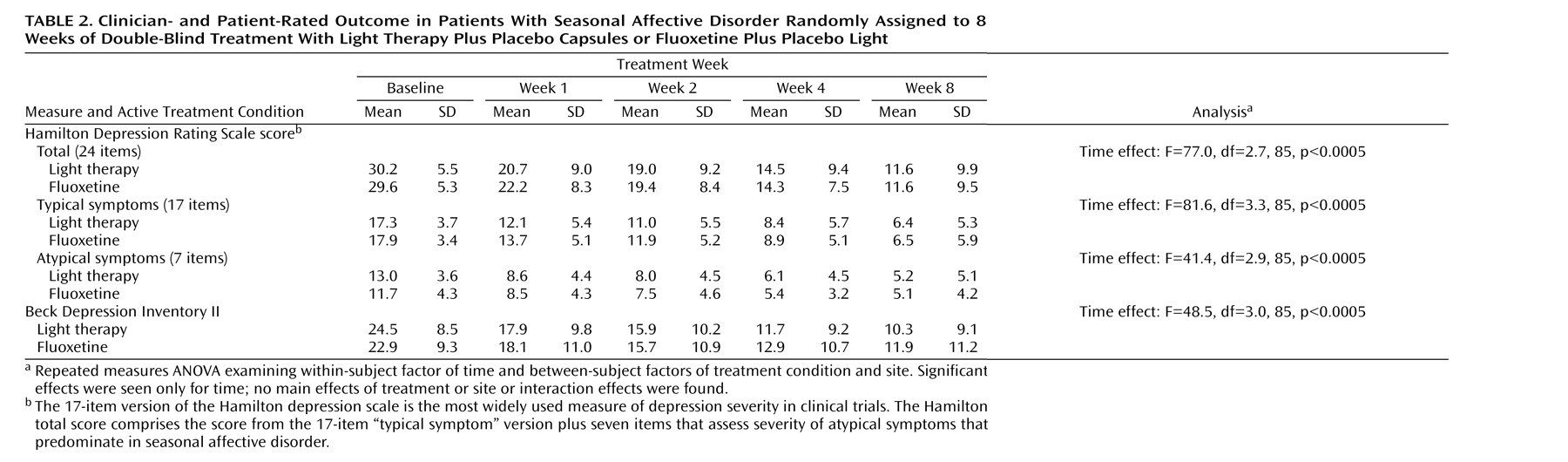
Table 2 shows the results of the repeated measures ANOVA for the primary outcome variable, the 24-item Hamilton scale, and for the “typical” and “atypical” symptoms as represented by the 17-item Hamilton scale and the seven-item atypical symptom addendum. Significant main effects of time were found for all comparisons, but no main effects were seen for treatment condition or site. There were no significant interaction effects for condition-by-time or condition-by-time-by-site. The same findings held for the patient-rated measure, the Beck Depression Inventory II. These analyses show that both groups improved on all measures over time but that there were no differences in the responses to the two conditions or between the four sites. Another analysis showed no differences in outcome measures with month of entry into the study (data not shown).
There were no significant differences between light treatment and fluoxetine in the clinical response rate (67% for both conditions) (χ 2 =0, df=1, p=1.00) or the clinical remission rate (50% versus 54%, respectively) (χ 2 =0.04, df=1, p=0.84). Similarly, there were no significant differences between conditions in the CGI improvement rating at last visit (mean=1.90 [SD=1.15] versus 1.92 [SD=1.09], respectively) (t=0.09, df=94, p=0.93). Another measure of clinical response is the percentage of patients with “much improved” or “very much improved” CGI ratings; again, there were no differences between conditions on this measure (73% for both groups) (χ 2 =0, df=1, p=1.00).
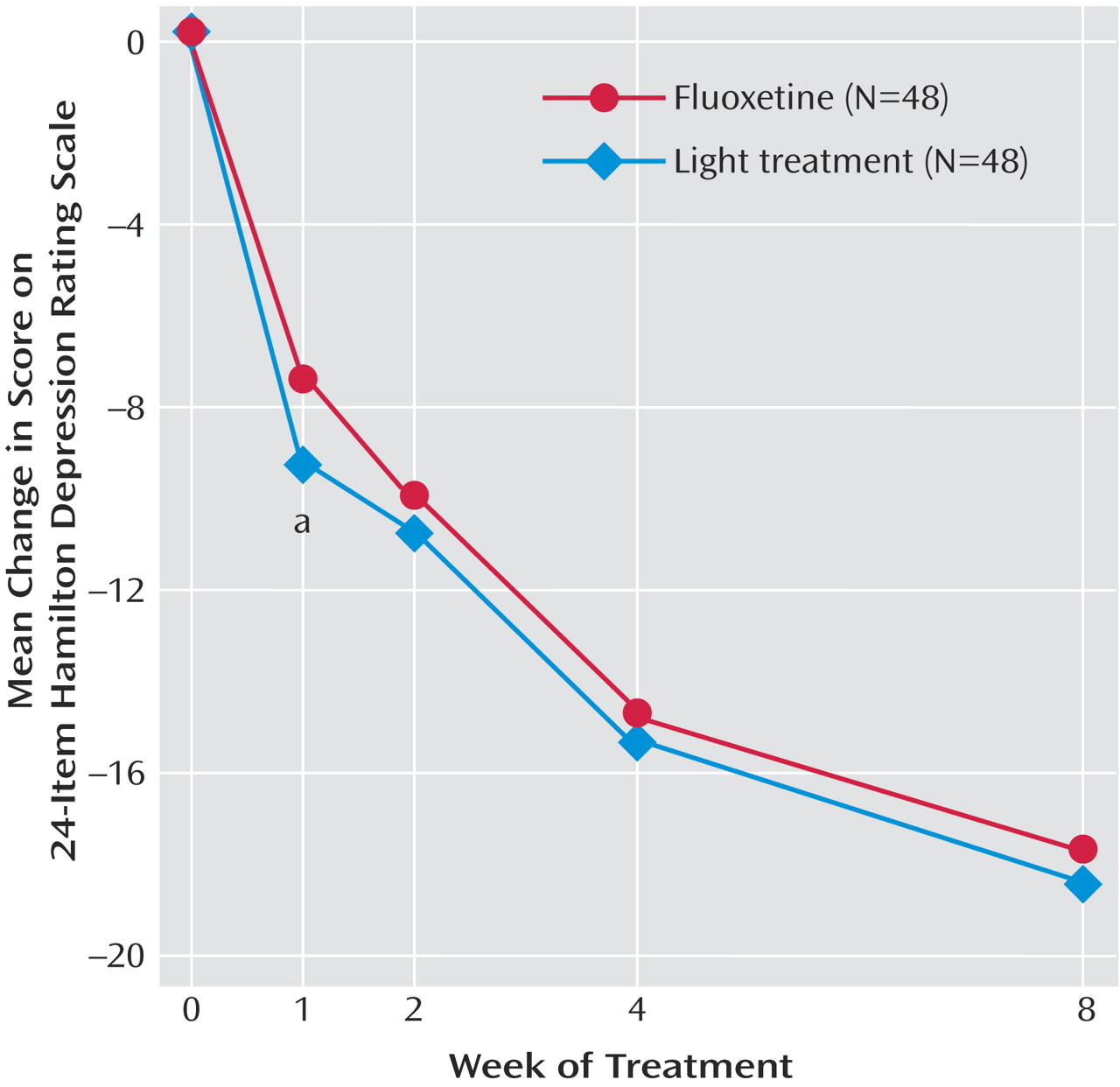
Figure 2 shows the changes in 24-item Hamilton depression scale scores from baseline for each week of treatment. Post hoc t tests showed that patients in the bright light condition improved more in the first week of treatment relative to those in the fluoxetine condition. However, the subsequent treatment weeks showed no differences between the two conditions. The effect size found in this study for the 24-item Hamilton change score between treatments at the final visit was 0.03 in favor of light treatment, indicating a trivial difference between conditions.
A subset of patients (N=49) was identified as being more severely depressed at baseline (24-item Hamilton depression scale scores ≥30). In this more severely ill subgroup, there were no significant differences in the 24-item Hamilton depression scale scores between the light-treated patients (N=27) and the fluoxetine-treated patients (N=22). The results again showed a significant main effect for time (F=49.2, df=2.9, 39, p<0.0005), but no other significant main effects or interaction effects were found. Similarly, in these more severely ill patients, there were no significant differences between light treatment and fluoxetine in the clinical response rates (70% versus 73%, respectively) (χ 2 =0, df=1, p=1.00) or the remission rates (48% versus 50%, respectively) (χ 2 =0, df=1, p=1.00).
The percentage of patients experiencing at least one treatment-emergent adverse event was 77% for light treatment and 75% for fluoxetine.
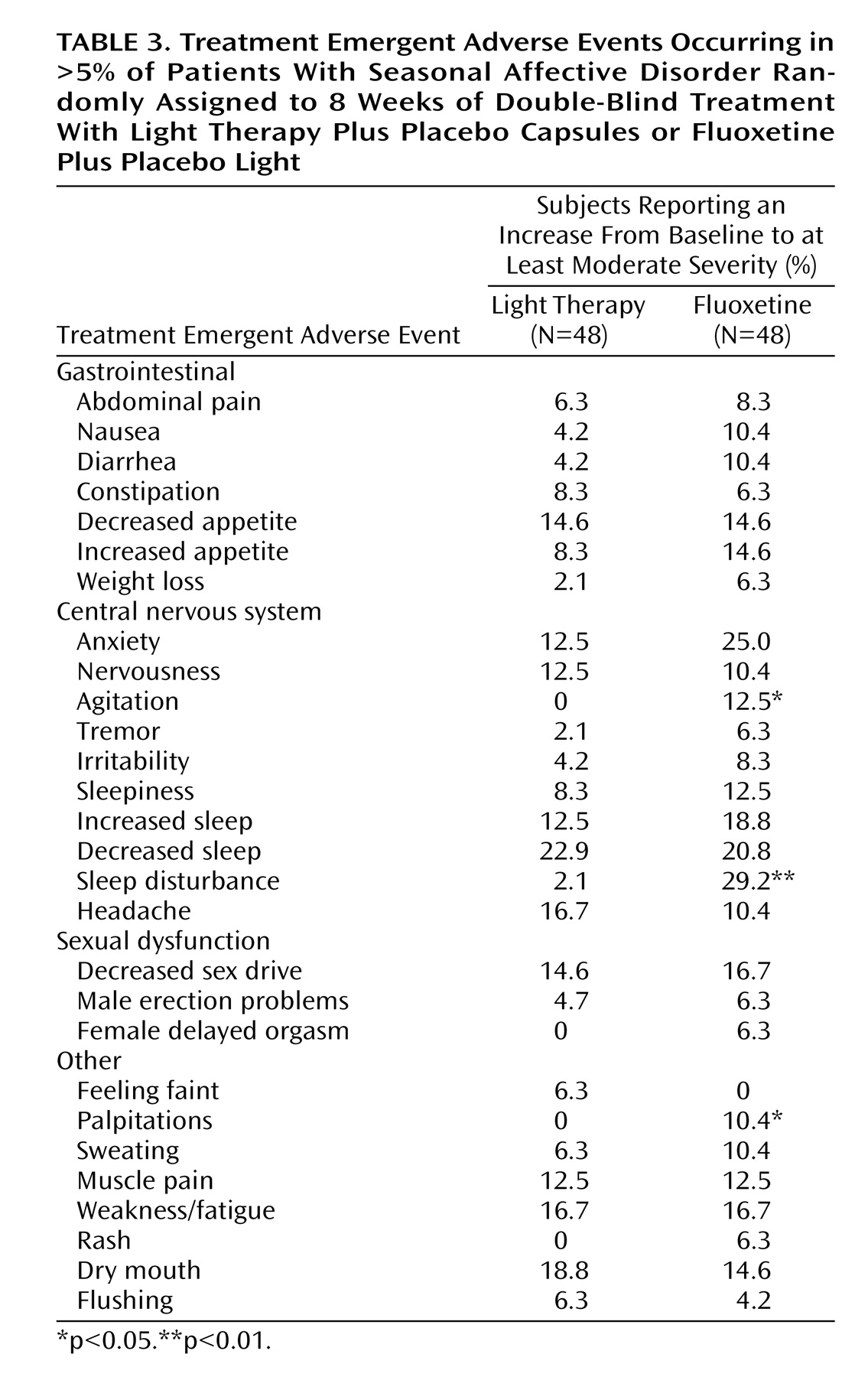
Table 3 shows the treatment-emergent adverse events reported in more than 5% of patients for either condition. Significant differences in treatment-emergent adverse events, all higher in the fluoxetine-treated patients, were found for agitation, sleep disturbance, and palpitations. There were no switches to hypomania with either treatment. The percentage of patients reporting at least one treatment-emergent adverse events self-rated as “severe” at least once during treatment was 33.3% for bright light and 35.4% for fluoxetine (χ
2 =0, df=1, p=1.00). Similarly, there were no significant differences between light and fluoxetine treatment in overall dropout rates in the study (8 versus 7, respectively; χ
2 =0, df=1, p=1.00) or in dropouts due to treatment-emergent adverse events (N=1 and 2, respectively; χ
2 =0, df=1, p=1.00).
Discussion
The main results of this study showed that there were no differences in the primary or secondary outcomes for patients with seasonal affective disorder who received active light therapy versus those who received fluoxetine. The clinical response and remission rates for light therapy and fluoxetine were very similar throughout the study. The benefits of treatment were apparent in both interviewer-rated and patient-rated outcome scales and in the typical and atypical symptom subscales. The light-treated patients had a greater improvement after 1 week of treatment (a finding which, given the post hoc nature of the analysis, needs to be regarded with caution), but thereafter there were no differences between conditions. Another caveat is that the study was only powered to detect medium-sized or larger effects. However, the actual differences between treatments in this study were so small that a huge sample (numbering in the thousands of patients) would be required to detect these effect sizes. Even if statistically significant, these small effect sizes would not be clinically meaningful.
This trial is the longest controlled study of light therapy. At 8 weeks, it is the only one of similar duration to the standard antidepressant clinical trial. Given the natural course of seasonal affective disorder with spontaneous clinical remission in the spring/summer, it is important for a longer-duration study to start treatment well before the time of spontaneous remission
(18) . In this study, there were no differences in outcome with month of entry, indicating that spontaneous remissions were avoided with patients entered in the later months. We also used a higher entry score on the 24-item Hamilton depression scale than other seasonal affective disorder studies, ensuring that patients were at least moderately depressed before treatment assignment.
Comparing light therapy and antidepressant medication is difficult because of differences in procedure and expectations with the two treatments. Light therapy has a behavioral component in that patients must wake up and spend 30 minutes in quiet activity while receiving light. The finding that expectation ratings were higher for light therapy than for medication confirms that it was also important to control for positive expectation effects of light treatment. We chose to use the “double dummy treatment” method to control for these nonspecific effects by having patients use both a light device and a pill. “Dim” light of 100 lux is still plausible to patients but is no brighter than ordinary kitchen lighting that patients might experience in their own homes. The deception was effective because there were no differences in pretreatment expectations for light between the groups even after patients were shown their assigned light box. Regardless, because 100-lux light may have some biological activity under certain conditions
(26), we cannot exclude the possibility that the dim light may have some active antidepressant effect.
Another limitation of this study is that a “double placebo” was not used, so we cannot rule out placebo effects (or the effects of waking up early) in the overall responses to both treatments. Given that both light therapy and fluoxetine have evidence for efficacy versus placebo, we believed that an effectiveness study (comparing two active treatments) would be adequate to answer the clinical questions. Reassuringly, the response and remission rates for light and fluoxetine in this study were similar in magnitude to those reported in placebo-controlled trials.
Of interest is that the response to light therapy in this study was not as rapid as reported in other shorter-term light studies. Patients showed steady improvement during the course of the 8 weeks, a pattern more similar to a medication effect. A possible explanation is that different expectation effects for a shorter-term study may contribute to the rapid response
(27) .
This study was a comparison of fixed-dose strategies. It is possible that the response rates would be higher if higher dosing was used. However, fluoxetine has a flat dose-response curve so that studies comparing higher fluoxetine doses do not show greater response
(28 –
30) . As for light therapy, there have been few studies of optimal “dosing” of light
(31) . The morning timing of light therapy followed standard practice based on results from randomized controlled trials and meta-analyses. However, one meta-analysis found that morning plus evening light exposure was more effective than exposure at a single time of day
(32) . Another study suggested that light therapy is most effective if applied at an optimal time in the circadian phase of the patient
(33), which may be at different external clock times for individual patients. Although this latter finding has yet to be replicated, if true, then the morning timing of light in this study may not have been optimal for every patient.
Some previous studies have suggested that fluoxetine is more effective than placebo in more severely ill patients
(18), while light therapy is more effective in less severely ill patients
(8) . In our study, just over half of the patients were considered to be more severely depressed, i.e., with a baseline score ≥30 on the 24-item Hamilton depression scale. In the post hoc analysis for this subset of patients, there was a good response to both treatments, but no differences were seen between treatments in improvement in depression scores or in clinical response or remission rates. Therefore, both light therapy and fluoxetine are comparably effective, even in more severely ill outpatients.
Fluoxetine was associated with a greater frequency of some treatment-emergent adverse events (agitation, sleep disturbance, palpitations) than light therapy, but both treatments were generally well tolerated, and there were no differences in severe treatment-emergent adverse events or dropouts due to adverse events. The higher rate of treatment-emergent adverse events reported in this study is likely related to the use of a self-rated adverse events scale instead of relying on unsystematic and spontaneous reports of adverse events as per usual in clinical trials. Despite this more intensive approach, the rates of many treatment-emergent adverse events, such as sexual dysfunction, were low (less than 5%) for both light therapy and fluoxetine. Adherence to treatment was also good in this study, as evidenced by patient logs and pill counts. However, studies have suggested that objective measures of adherence with light therapy usually show lower adherence rates than subjective patient reports
(34) .
In summary, light therapy and fluoxetine are comparably effective treatments for patients with seasonal affective disorder, although light treatment may have a slightly faster onset of effect and slightly fewer treatment-emergent adverse events. The choice of treatment always depends on individual assessment of risks and benefits
(15), but in the absence of clear superiority for either treatment, patient preference should be a major factor in treatment selection. Predictive factors (symptoms, personality traits, circadian measures) of response to light or medication, and differences in quality of life and cost-benefit of treatments, also should be of interest; analyses of other data from this study addressing these issues are in progress. Future seasonal affective disorder studies should also examine the combination of light therapy and antidepressants for nonresponders to monotherapy.