Night eating syndrome is an eating disorder characterized by morning anorexia, evening hyperphagia, and insomnia with awakenings followed by nocturnal ingestions
(1,
2) . In addition, mood is usually low
(3), with a pattern of worsening in the latter half of the day
(2) . The core feature of night eating syndrome appears to be delay in the circadian timing of food intake
(4) . Food intake is lower in the first half of the day and greater in the evening and nighttime. Sleep is often disrupted in the service of food ingestion. In the largest controlled study to date of overweight and obese outpatients with night eating syndrome
(4), energy intake in the first 8 hours of the day (6:00 a.m. to 2:00 p.m.) averaged only 575 kcal in night eating syndrome subjects (N=46) versus 1,082 kcal in a comparison group (N=43), whereas energy intake in the last 8 hours (10:00 p.m. to 6:00 a.m.) averaged 591 kcal in night eating syndrome subjects versus only 118 kcal in comparison subjects. The total energy intake over 24 hours was not different between the two groups.
Night eating syndrome is of clinical importance because it is associated with both obesity and psychological distress. Its prevalence has been estimated at 1.5% in the general population
(5) with a reported range of 8.9%
(6) to 14%
(3) in obesity clinics and rates of up to 27% in severely obese persons
(5) . Night eating syndrome appears to be more common in obese persons than in nonobese persons and to increase in prevalence with increasing adiposity. In a Danish study
(7), female obese subjects exhibiting night eating gained 5 kg over a 6-year period, whereas female obese subjects who did not engage in night eating gained 1 kg. About half of individuals with night eating syndrome report that they were of normal weight before the syndrome developed, suggesting that night eating syndrome may be an important pathway to obesity
(8) .
There have been few reports on the treatment of night eating syndrome. Case reports have suggested benefit from a variety of strategies including
d -fenfluramine
(9), phototherapy
(10), progressive muscular relaxation
(11), and topiramate
(12) . The first clinical pharmacotherapy trial
(13) was a 12-week, open-label study of 17 subjects treated with sertraline, a selective serotonin reuptake inhibitor (SSRI). A significant reduction of symptoms was seen in obese subjects with night eating syndrome, with about half (N=8) of the group responding to sertraline. Those responders who achieved remission of night eating syndrome (N=5) also lost a significant amount of weight (–4.8 kg, SD=2.6).
The present study sought to follow up this previous open-label trial with a double-blind, randomized, placebo-controlled trial. This time we also included a small number of normal weight subjects with night eating syndrome to determine if sertraline might relieve the distress associated with the syndrome.
Method
Subjects were recruited from a study that characterized the psychological and behavioral aspects of night eating syndrome
(4) . These patients were recruited through a combination of print advertisements, TV programming, and a website. The characterization study included 1) a structured clinical interview designed to assess the presence or absence of night eating syndrome, performed by a trained clinician; 2) a 10-day sleep and food diary; 3) the Structured Clinical Interview for DSM-IV (SCID) to assess the presence of past or current psychiatric disorders; and 4) the Eating Disorder Examination to assess the presence of concomitant eating disorders.
Participants
Eligible subjects were at least 18 years of age, met standard criteria for night eating syndrome according to the structured clinical interview, and had a body mass index (BMI) >18 kg/m 2 . Applicants were excluded if they 1) were severely depressed (symptoms in excess of the number required for DSM-IV diagnosis and markedly interfering with occupational functioning or with usual social activities or relationships); 2) had a lifetime diagnosis of bipolar disorder or any psychotic disorder; 3) reported substance abuse or dependence within the preceding 6 months; 4) were currently taking psychotropic medications (including hypnotics); 5) were working a night shift or swing shift schedule; 6) were in a weight reduction program; 7) had a current diagnosis of anorexia nervosa or bulimia nervosa (but not binge eating disorder); or 8) lacked awareness of their night eating episodes. The latter criterion was used to exclude subjects with nocturnal sleep-related eating disorder, a parasomnia in which nocturnal eating is accompanied by a lack of awareness at the time and subsequent amnesia for the behavior.
Procedures and Measures
Baseline night eating syndrome symptoms were assessed as part of the characterization of night eating syndrome study
(4) . Each subject collected data in a food and sleep diary during a 10-day 24-hour prospective monitoring period, with the first 2 days discarded as practice days and the last day discarded because of incomplete data. The diary included a record of all meals, snacks, and beverages consumed. Awakenings (during which the subject got out of bed), nocturnal ingestions, as well as the timing of bedtime and morning awakening were all recorded. A research dietician analyzed diaries for caloric intake and macronutrient content. Subjects were paid for the baseline diary data collection but not for participation in the treatment trial itself. Of the 65 subjects with night eating syndrome who completed the diary assessment, 28 who were eligible to participate in the trial decided not to. Reasons for not participating were inability to schedule (N=16), not wanting to be in a placebo-controlled study (N=7), and not wanting a medication (N=5). This left a total of 37 subjects who entered the treatment trial. Three subjects attended the baseline visit but did not return for any subsequent visits, leaving a total of 34 subjects whose data were included in the analyses.
Subjects were randomly assigned to 8 weeks of double-blind treatment with sertraline or placebo. Psychotropic agents other than the study medication were prohibited during the study. The 8-week duration of the trial was based on the results of our earlier 12-week trial. Subjects took tablets, identical in appearance, containing either 50 mg of sertraline or placebo. Subjects commenced with one tablet daily taken with the evening meal. Subjects were seen every other week for 30-minute visits at which time medication dosage could be adjusted up to a maximum of four tablets daily. Medication tolerance and adherence were recorded at each visit.
Subjects were weighed at each visit and completed three self-report scales: 1) a night eating symptom scale, 2) the Beck Depression Inventory, and 3) the Quality of Life Enjoyment and Satisfaction Questionnaire. The night eating symptom scale is a self-report scale measuring the range and severity of night eating symptoms over the preceding week
(13) . It measures in a series of 13 items the degree of morning anorexia, evening hyperphagia, sleep disturbance, nocturnal eating episodes and associated cravings or compulsion to eat, and level and pattern of mood disturbance. Each item is scored from 0 to 4, providing a possible range of scores from 0 to 52. The study physician recorded the number of nighttime awakenings (defined as when the subject got up out of bed for reasons other than solely to use the bathroom) and ingestions. The study physician also administered the Clinical Global Impression (CGI) improvement and severity scales and the 17-item Hamilton Depression Rating Scale at each visit. The Hamilton and Beck instruments were used to track changes in depressive symptoms. Outcome was categorized at week 8 on the basis of the CGI improvement rating, which ranges from 1 to 7. CGI improvement ratings were considered a primary outcome measure, with a priori standards applied as follows: subjects with scores of 2 (much improved) were categorized as having responded, and those with scores of 1 (very much improved) were categorized as having remitted.
Evening hyperphagia was assessed by reviewing with the subject the proportion of their daily caloric intake that occurred between the end of the evening meal and bedtime plus any nocturnal ingestions that occurred. As some subjects with night eating syndrome delay their evening meal considerably as part of the circadian delay in the food intake rhythm, a cutoff of 8:00 p.m. was used. Any food intake commencing after this time was considered to be caloric intake after the evening meal. The total caloric intake after the evening meal represents the sum of calories ingested between the end of the evening meal and bedtime plus any calories derived from nocturnal ingestions; it is expressed as a percentage of total 24-hour calorie intake.
The Institutional Review Board of the University of Pennsylvania approved the protocol. All subjects signed the informed consent form after study procedures had been fully explained.
Data Analysis
Means and standard deviations of the outcome variables at each time point were used for descriptive statistics as well as for the depiction of trends over the 8 weeks. The analyses were conducted on an intent-to-treat basis for all subjects who completed at least one follow-up visit after the baseline visit. The repeated-measures outcome variables over the 8-week period were analyzed by a mixed effects linear regression model in the following form: outcome variable=intercept + group + week + group x week. The intercept was assumed to be random in order to take within-subject correlations of the dependent variables into account for statistical inference. Group and time variables were taken as fixed and discrete; two-sided p<0.05 was considered significant. This mixed effects modeling approach with available cases using a maximum likelihood method is valid in the presence of observations missing at random
(14) .
The effects of particular interest were the main group effect and the group-by-week interaction, with this interaction representing differences in trends of the outcome variables over time between night eating syndrome and comparison groups. Omnibus interaction significance tests are reported in the text. Post hoc testing of the main group effects at each time point on the outcome variables was followed by testing of the corresponding parameter contrasts in the mixed effects model with Wald t tests. For this purpose, we used a Bonferroni-corrected significance level 0.05/4=0.0125 (correcting for the number of treatment visits after baseline), except when testing for correlations. Main group effects at any week with p values lower than this corrected significance level were declared to be significant even if the overall main group or interaction effects were not significant.
Three subjects who met criteria for binge eating disorder in addition to night eating syndrome were included as part of the sample. In line with recent evidence, binge eating disorder and night eating syndrome appear to be two distinct disorders rather than variable expressions of the same underlying psychopathology
(15,
16) . In this respect, binge eating disorder was treated as a comorbid condition in night eating syndrome subjects and was not viewed as exclusionary for study participation. All three subjects with binge eating disorder plus night eating syndrome were randomly assigned to the placebo group. We tested for the significance of binge eating disorder by including binge eating disorder status in the aforementioned mixed effects models. Binge eating disorder status did not have a significant effect on any of the outcomes except for caloric intake after the evening meal. Thus, caloric intake after the evening meal was the only variable for which binge eating disorder status was controlled. Pearson’s correlations were used to assess the association of changes at week 2 with those at week 8 in the SSRI group. We used SAS v8.2 for statistical analyses.
Results
Characteristics of the sertraline and placebo groups at baseline are presented in
Table 1 . No significant between-group differences for these variables were found.
CGI Ratings
The CGI improvement rating classified 12 of the 17 subjects receiving sertraline as having responded (score ≤2), and seven of these 12 achieved remission or complete resolution of night eating syndrome symptoms (F=6.7, df=4, 113, p<0.001). Of those receiving placebo, only three subjects were classified as having responded (a response rate significantly lower than that seen with sertraline [χ 2 =9.66, df=1, p<0.002]), with one of the three placebo responders achieving remission status. Of the three normal weight subjects in the sertraline group, two responded, while none of the three normal weight subjects in the placebo group responded.
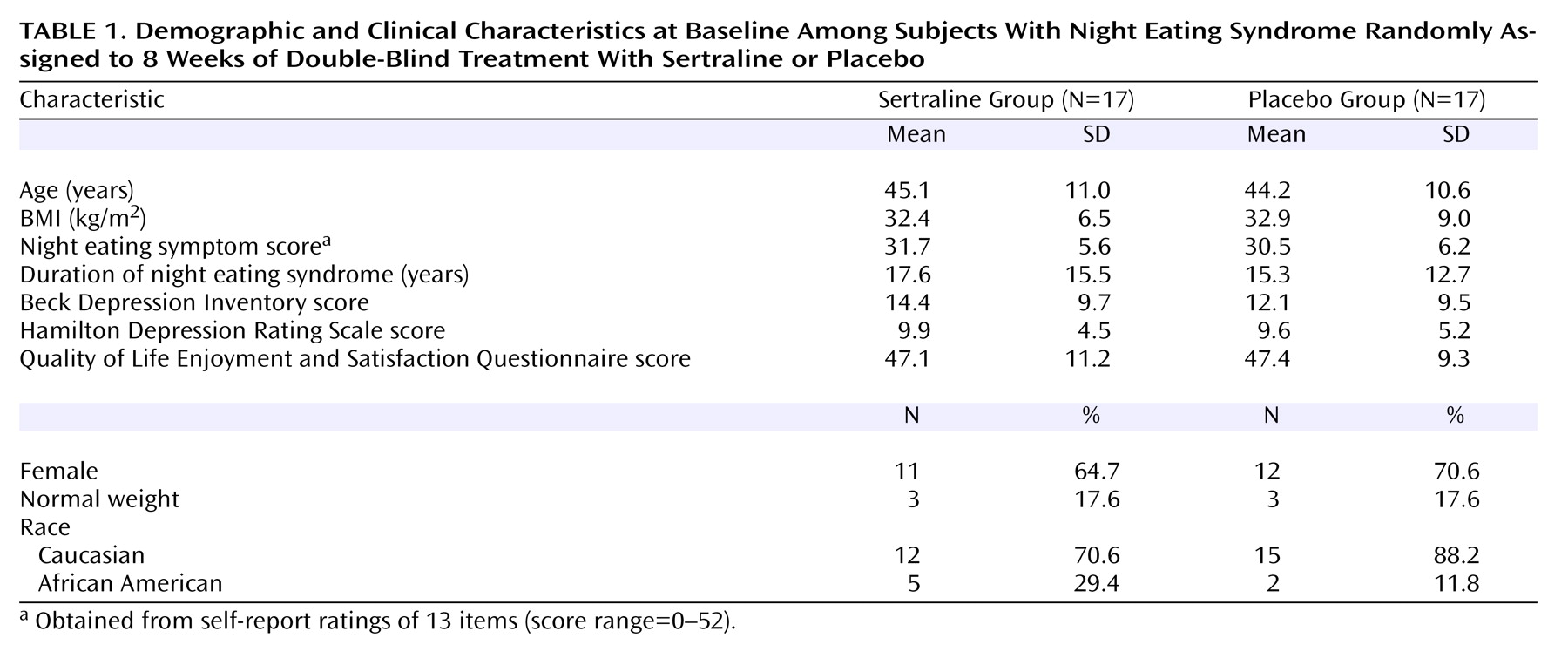
Figure 1 shows that the largest reduction in symptoms occurred between baseline and week 2, indicating an early and robust effect of sertraline. Overall, a subject receiving sertraline had a 30% chance of responding by week 2. Five of the 12 who ultimately responded to sertraline had responded as early as week 2, and four of these five achieved remission status by week 2. The lack of early improvement with sertraline did not preclude ultimate response, as 50% of all responses occurred between weeks 4 and 8.
The CGI severity scale is a further index of overall change in night eating syndrome symptoms. The sertraline group had a reduction of two points in symptom severity, from 4.2 at baseline (moderate severity) to 2.2 at endpoint (borderline ill), whereas there was a much more modest reduction (from 4.2 to 3.4) in the placebo group (F=4.1, df=4, 107, p=0.004).
Night Eating Symptoms
Changes in night eating syndrome symptoms were significantly greater in the sertraline group, as assessed by night eating symptom scores over the course of the 8-week study (
Figure 1 ).
By week 8, the night eating symptom scores of the sertraline group had dropped by 18.1 points (57%) from a baseline score of 31.7 as compared with a reduction of only 5 points (16%) from a baseline score of 30.5 in the placebo group (F=8.0, df=4, 112, p<0.0001). A significant correlation was found between the change in night eating symptom scores from baseline to week 2 and the change from baseline to week 8 for subjects receiving sertraline (r=0.68, p=0.01), indicating that early improvement with sertraline was predictive of ultimate response. In addition, in terms of the speed of response, the dose at first observed response in the sertraline group was correlated with the week of response, suggesting that those responding early improved at lower doses than those responding later (r=0.84, p<0.001). However, the probability of response by the study endpoint at week 8 was not correlated with dose, indicating that dose per se was not an important predictor of ultimate response to sertraline (r=0.52, p<0.09).
Ingestions and Awakenings
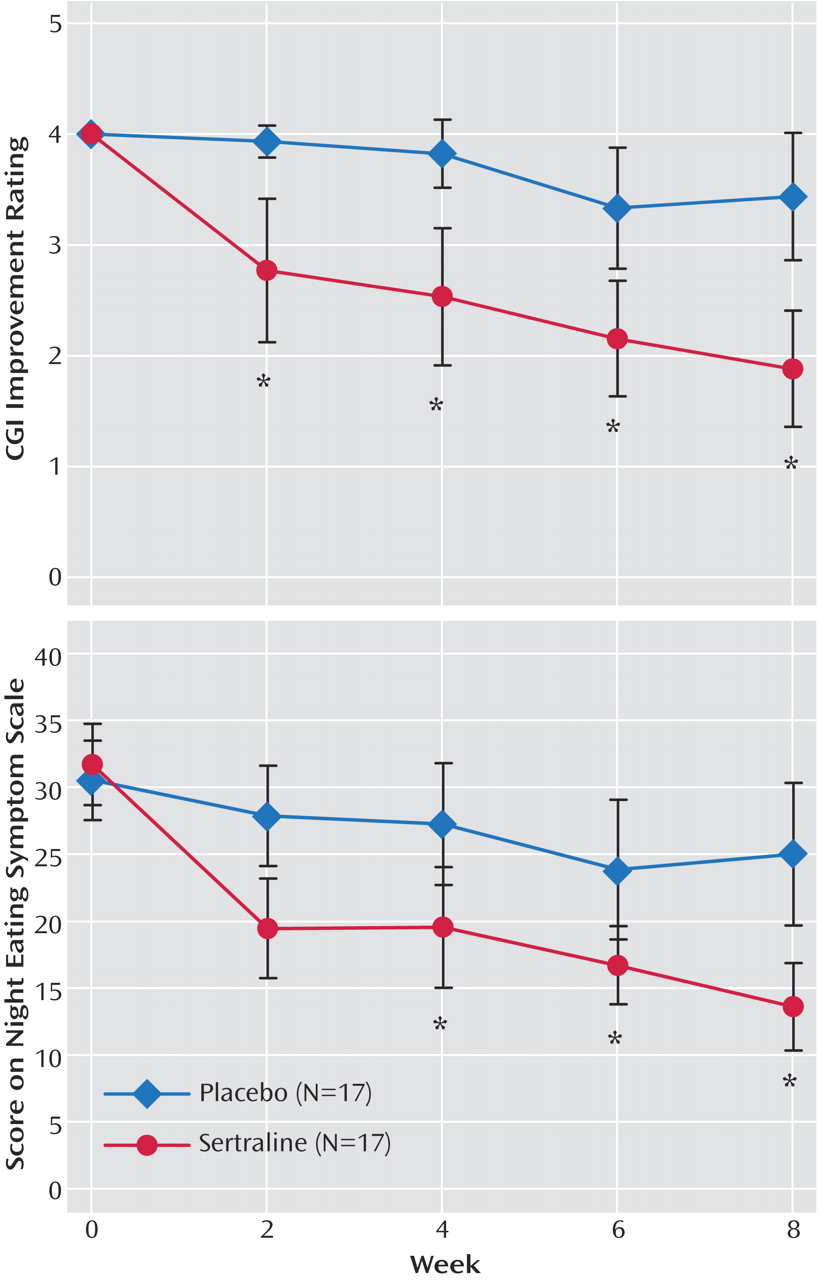
Figure 2 shows a significant reduction in the frequency of nocturnal ingestions in the sertraline group relative to the placebo group. The number of nocturnal ingestions in the sertraline group fell by 81% (from a mean at baseline of 8.3 per week [SD=8.5] to 1.6 [SD=2.6]) versus a fall of only 14% for the placebo group (from 6.4 [SD=4.9] to 5.5 [SD=4.9] per week) (F=3.7, df=4, 80, p=0.01).
Figure 2 indicates that the number of awakenings fell by 74% in the sertraline group (from a mean of 8.8 per week [SD=8.6] to 2.3 [SD=4.7]) versus a fall of only 14% in the placebo group (from 6.4 [SD=4.6] to 5.5 [SD=5.0]). This drop failed to reach significance in the overall interaction effect (F=0.9, df=4, 80, p=0.40), but it yielded a difference in main effect between groups (F=4.7, df=1, 32, p=0.03). In post hoc testing, after adjustment for multiple comparisons, the difference at week 8 was not significant (t=–2.52, df=80, p=0.0137).
Caloric Intake After the Evening Meal
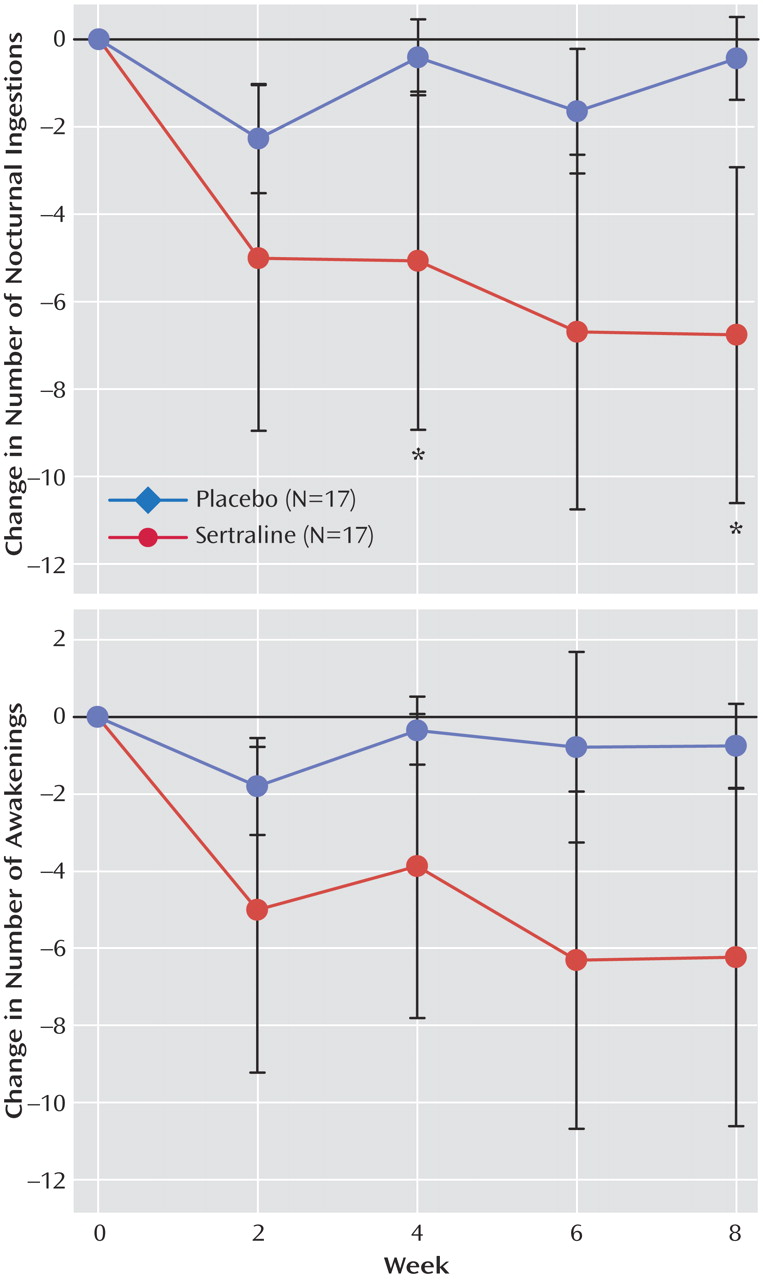
Figure 3 shows that caloric intake after the evening meal in the sertraline group fell by 68%, from 47.3% of total daily calories at baseline to 14.8% at week 8. In the placebo group, caloric intake after the evening meal fell by 29.3%, from 44.7% at baseline to 31.6% at week 8 (F=3.5, df=4, 106, p=0.009). Comparisons of individual time points were not significant (week 8: t=2.0, df=106, p=0.047).
Weight Change
Among overweight subjects (N=14 in both groups), the sertraline group lost 2.9 kg (SD=3.8) versus 0.3 kg (SD=2.7) in the placebo group (F=2.6, df=4, 63, p=0.06). The difference in main effect for weight between groups at week 8 was significant (t=–2.7, df=63, p=0.009). The three normal weight subjects receiving sertraline lost 1.2 kg compared with a gain of 0.3 kg by the three normal weight subjects receiving placebo.
Mood Measures
Mood measures showed only a modest level of depressive symptoms in both groups at baseline (
Table 1 ), and they did not differ over time (Hamilton score change: F=1.5, df=4, 110, p=0.20; Beck score change: F=1.9, df=4, 100, p=0.10).
Change in night eating symptom scores in the sertraline group did not significantly correlate with reduction in depressive symptoms as assessed with either the Beck Depression Inventory (r=0.26) or Hamilton depression scale (r=0.08). When the two depression items were removed from the full night eating symptom scale, the score on the modified scale still correlated strongly with the full scale score (r=0.98, p<0.001), implying that change in depressive symptoms was not the principal driver of change in night eating syndrome symptoms.
Quality of Life
In the sertraline group there was an increase in score on the Quality of Life Enjoyment and Satisfaction Questionnaire, from 47.1 (SD=12.0) at baseline to 54.3 (SD=9.6) at week 8. Those receiving placebo remained essentially unchanged (mean=47.6 [SD=9.9] at baseline and 47.4 [SD=7.3] at week 8; F=2.5, df=4, 108, p=0.045). No differences were noted at specific time points.
Dosing and Adverse Events
The mean daily dose of sertraline at study endpoint was 126.5 mg (SD=50.4). In contrast, the average dose of placebo attained would have translated to 173.5 mg (SD=40.0), indicating that dose was appropriately increased when a suboptimal response was observed (t=3.0, df=32, p=0.005). Sertraline was well tolerated, and no subject withdrew because of adverse events. Common side effects were mild and included dry mouth, fatigue, diminished libido, and sweating. Nausea as an adverse event was infrequent and transient (affecting two subjects receiving placebo and one receiving sertraline). There were two dropouts in the study, each related to lack of efficacy, one from the sertraline group at week 6, and one from the placebo group at week 4.
Discussion
The results of this study, the first randomized, placebo-controlled trial of sertraline in the treatment of night eating syndrome, are clear. Sertraline, an SSRI medication, reduced the symptoms of night eating syndrome, and most subjects (71%) met response criteria at the end of 8 weeks. The extent of improvement in core night eating syndrome symptoms was striking. The number of nocturnal ingestions in the sertraline group was reduced by about 80% by study endpoint. The caloric intake after the evening meal dropped from 47.3% of total daily calories consumed at baseline to 14.8% at week 8, thus approaching the normative levels of intake for obese comparison subjects without night eating syndrome found in our previous study
(4) .
Consistent with the reduction in evening and nocturnal hyperphagia are the weight losses (about a pound a week), which were similar to those in our earlier, open-label trial with sertraline
(13) . This finding is the more striking in that no advice or behavioral guidance regarding weight loss was given. It suggests that sertraline may have a restraining effect on the tendency to gain weight in persons with night eating syndrome.
Two patterns of improvement with sertraline were evident. Five subjects experienced an early and robust improvement with sertraline, meaning that close to half of those ultimately responding (N=12) exhibited this response after only 2 weeks of receiving active medication. Improvement in the seven other responders occurred more gradually, between weeks 4 and 8. The fact that there was only a weak, nonsignificant correlation between improvement in depressive symptoms among night eating syndrome subjects receiving sertraline and improvement in night eating symptoms strongly implies that the improvement with sertraline was independent of its antidepressant effect.
Subjects receiving sertraline had experienced night eating syndrome for a prolonged period of time (average duration of 17.6 years) before entering the study, but nevertheless, four of the five fast responders achieved full remission after only 2 weeks of sertraline treatment at a dose of 50 mg/day. This indicates that, despite chronicity of symptoms, a rapid and robust improvement is possible for some night eating syndrome patients. A similar finding has been reported in another eating disorder, bulimia nervosa. When treated with the SSRI fluoxetine, a significant reduction was noted in both the binge eating and vomiting episodes after a single week of active treatment
(17) . It is possible that the nocturnal ingestions in night eating syndrome, while not actual binges, share the psychological component of disinhibition with the binges of bulimia, and that serotonergic medications such as fluoxetine and sertraline have the potential to quickly ameliorate the loss of control present in both disorders.
As indicated earlier, the core feature of night eating syndrome appears to be a delay in the circadian timing of energy intake, with intake suppressed in the morning and increased in the evening and night. In an earlier study of carefully monitored outpatients with night eating syndrome and weight-matched comparison subjects
(4), we found dissociation between the sleep and eating rhythms in the night eating syndrome group, with a delay in the food intake rhythm but not in the sleep rhythm.
The maintenance of normal circadian rhythms is the task of the suprachiasmatic nucleus of the hypothalamus, and serotonergic neurons are known to have inputs into the suprachiasmatic nucleus
(18) . It is possible that sertraline may act by modulating suprachiasmatic nucleus function to restore a more normal food intake pattern in subjects with night eating syndrome. The suprachiasmatic nucleus may be a site of action in some individuals for promoting a rapid improvement in night eating syndrome symptoms.
Limitations of this study include its short duration and small size. Future studies of sertraline and other pharmacotherapy agents in treating night eating syndrome should determine if positive results are sustained over a longer term. If so, sertraline may be able to control both the core night eating syndrome symptoms and the obesity that is a frequent and distressing complication of the syndrome.