Positron emission tomography (PET) studies of adults with ADHD have revealed underactivation of anterior cingulate cortices associated with working memory
(5) and underactivation of the hippocampal gyrus and insula during decision making
(6) . Since tasks of motor response inhibition reveal performance deficits in ADHD
(7), functional magnetic resonance imaging (fMRI) studies of children with ADHD have focused upon associated frontostriatal networks
(8) and have shown prefrontal activation to be either reduced
(9 –
11) or increased
(12 –
14) as well as hypoactivation in the anterior cingulate and supplementary motor area
(9) and increased activation in temporal cortices
(9) . While reduced caudate activation in ADHD patients has been consistently found during motor inhibition
(10 –
13), more inconsistent outcomes may be related to small study group sizes or the use of block designs, which are more sensitive to performance differences than event-related designs.
A possible confound in existing imaging studies is the inclusion of patients with ADHD with long-term medication history
(15) . Methylphenidate may increase frontostriatal activation
(12) and alter frontostriatal dopamine transporter binding sites in patients with ADHD
(16) . Long-term changes of methylphenidate on dopamine function have been observed in frontal and striatal regions in animals
(17) and in humans as a consequence of chronic stimulant abuse
(18) .
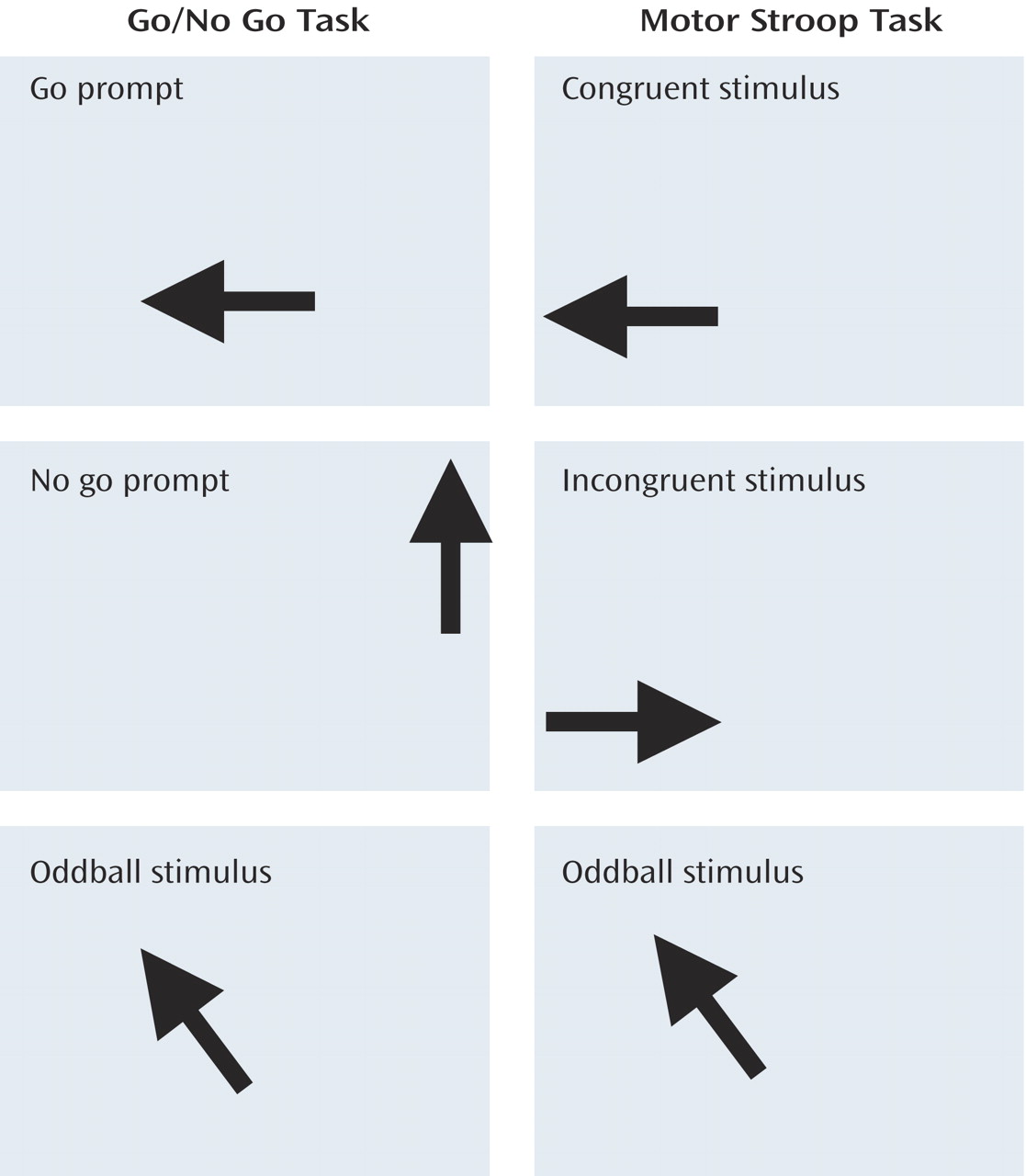
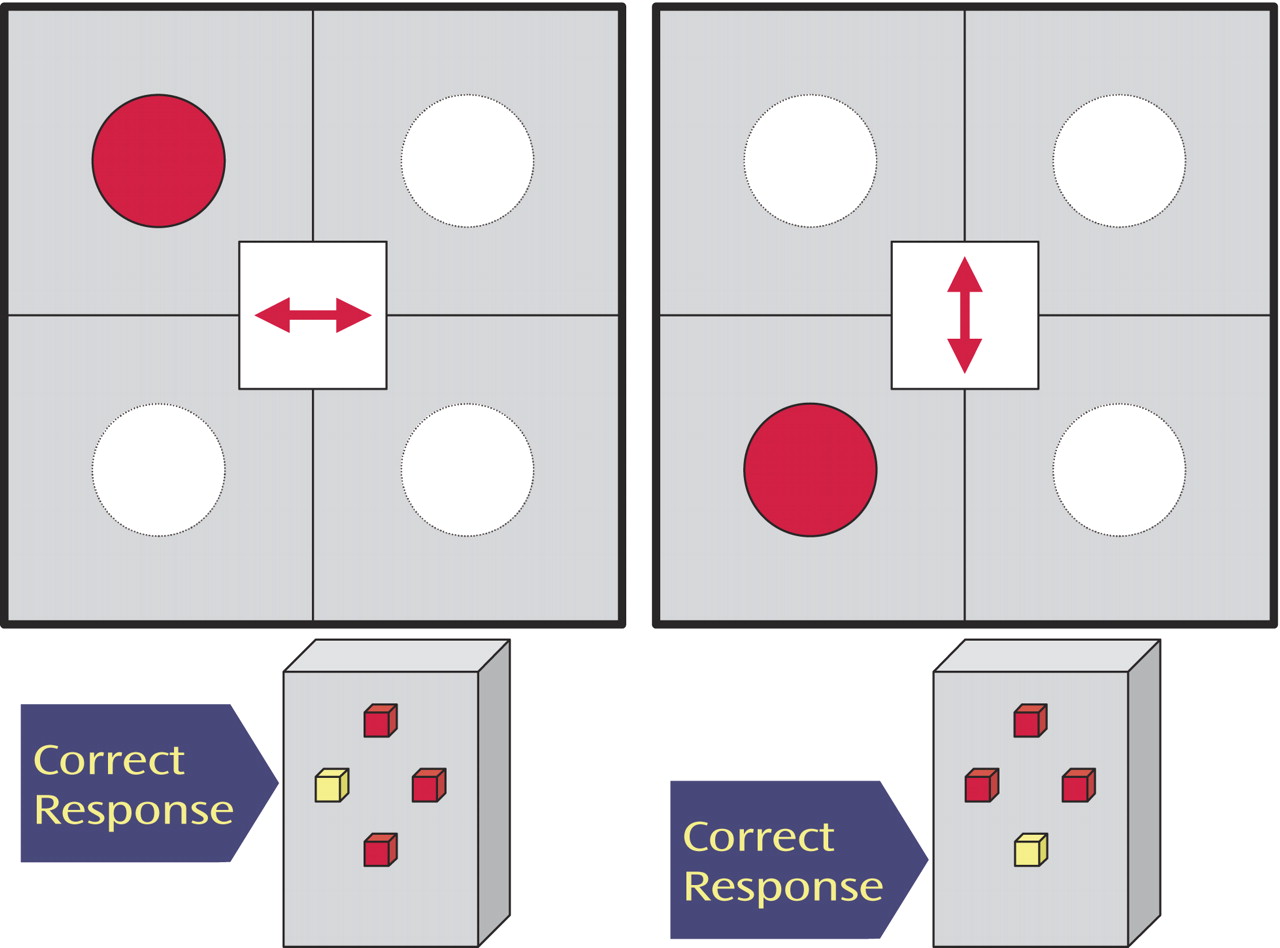
This study investigates whether neurofunctional abnormalities during inhibitory control can be observed in medication-naive children and adolescents with ADHD. We extended the probes from motor-inhibitory to more cognitive-inhibitory and attentional domains by using 1) a go/no go task to measure selective motor response inhibition, 2) a motor Stroop task to measure interference inhibition, and 3) a visual-spatial switch task to measure the ability to inhibit previously valid stimulus-response associations during switch events.
Children with ADHD have shown impairments in go/no go, Stroop, and switch tasks
(19), which are mediated by specific frontostriatal pathways thought to be impaired in ADHD children: mesial, orbitofrontal, and caudate regions for the go/no go task
(20) ; left inferior prefrontal and putamen areas for the motor Stroop task
(20) ; and right inferior prefrontal, caudate, and parietal regions for the switch task
(21) . However, no fMRI study has yet investigated interference inhibition in a Stroop-like task or cognitive switching in children and adolescents with ADHD, particularly with medication-naive patients.
We hypothesized that medication-naive boys with ADHD would show a reduction in task-specific prefrontal brain activation relative to healthy comparison subjects during frontal lobe-mediated tasks of motor and cognitive inhibition.
Results
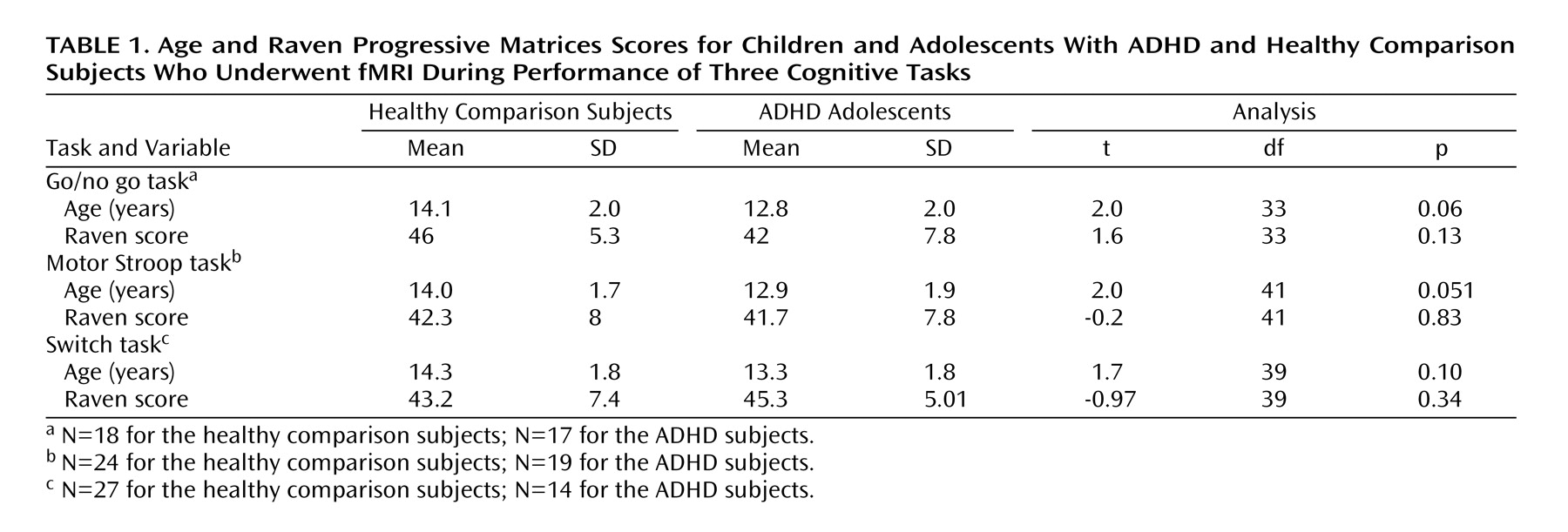
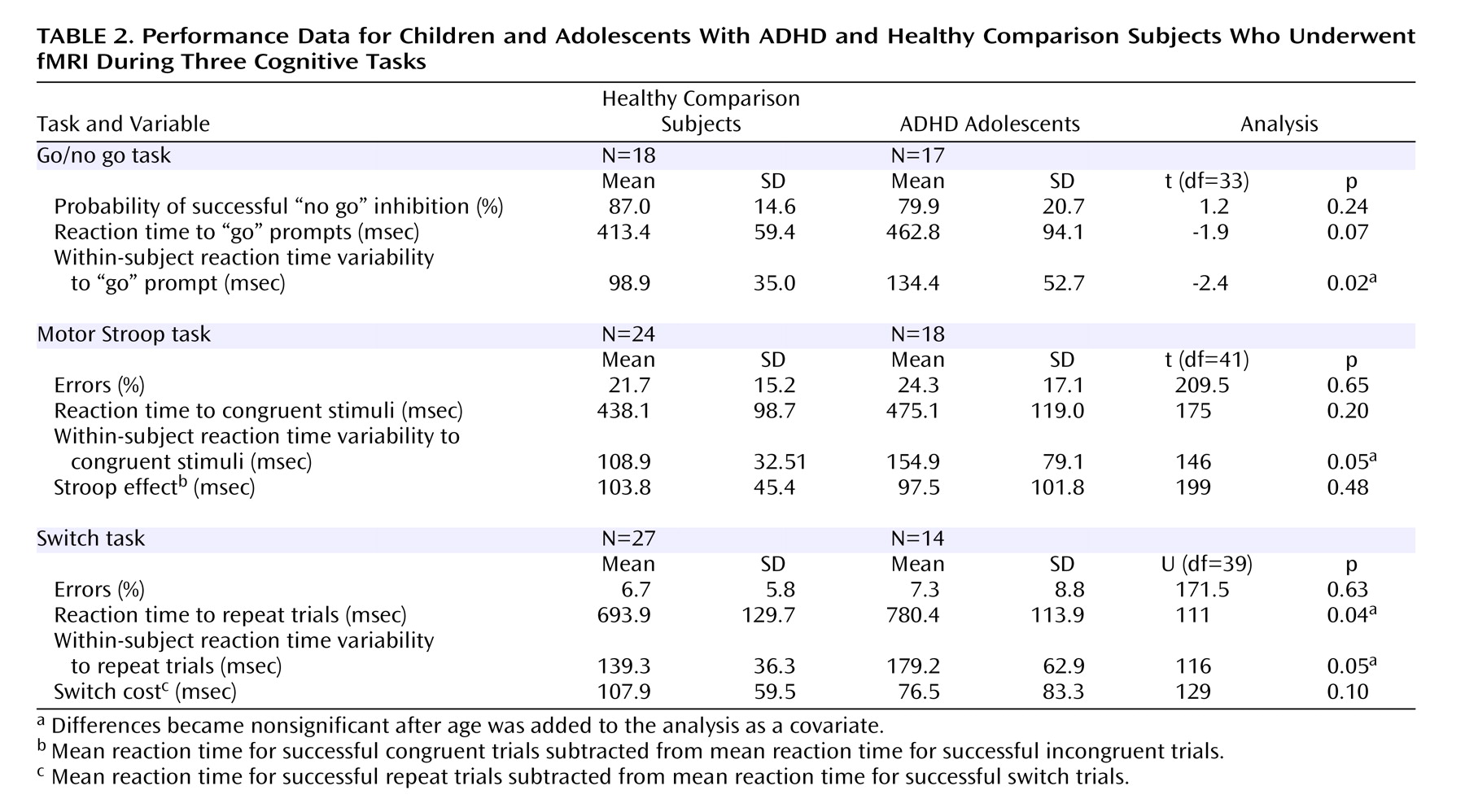
Performance Data
Each group’s performance data are shown in
Table 2 . Patients with ADHD had significantly slower reaction times to repeat trials in the switch task. The ADHD subjects also had significantly greater within-subject variability of reaction time to “go” prompts in the go/no go task, congruent stimuli in the motor Stroop task, and repeat trials in the switch task. No other group differences were observed.
Imaging Data
No significant group differences were observed in the extent of three-dimensional motion during any of the tasks.
Regions of activation for each group for each task as well as significant differences in activation between groups for each task are presented in a
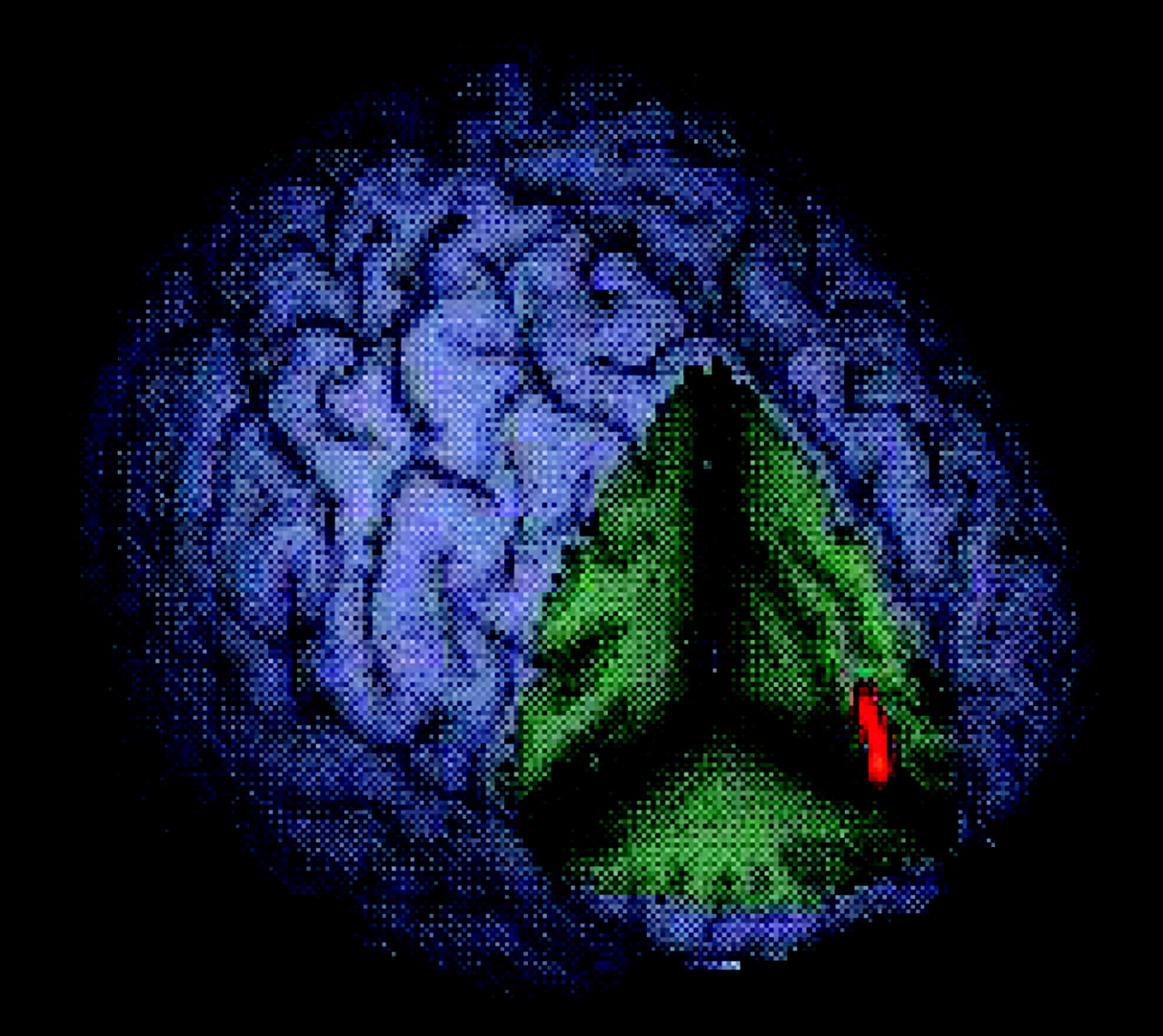
data supplement that accompanies the online version of this article. Significant activation associated with the no go/oddball contrast for healthy subjects included the mesial frontal and right inferior prefrontal cortex, anterior cingulate, right caudate, left temporal cortex, precuneus, bilateral insula, and right sensorimotor cortex. Boys with ADHD activated right superior and left inferior prefrontal cortices, left temporal cortex, and cerebellum. An ANCOVA revealed significantly greater activation in healthy subjects of left rostral mesial frontal cortex during successful no go versus oddball trials (
Figure 3 ).
The Stroop/oddball contrast in the healthy subjects revealed activation of the right dorsolateral prefrontal cortex, left inferior frontal cortices, right sensorimotor cortex, left temporal cortex, and bilateral cerebellum. Boys with ADHD activated the right inferior frontal and middle temporal cortices, left sensorimotor cortex, caudate, thalamus, and cerebellum. An ANCOVA revealed no significant group differences in activation for this contrast.
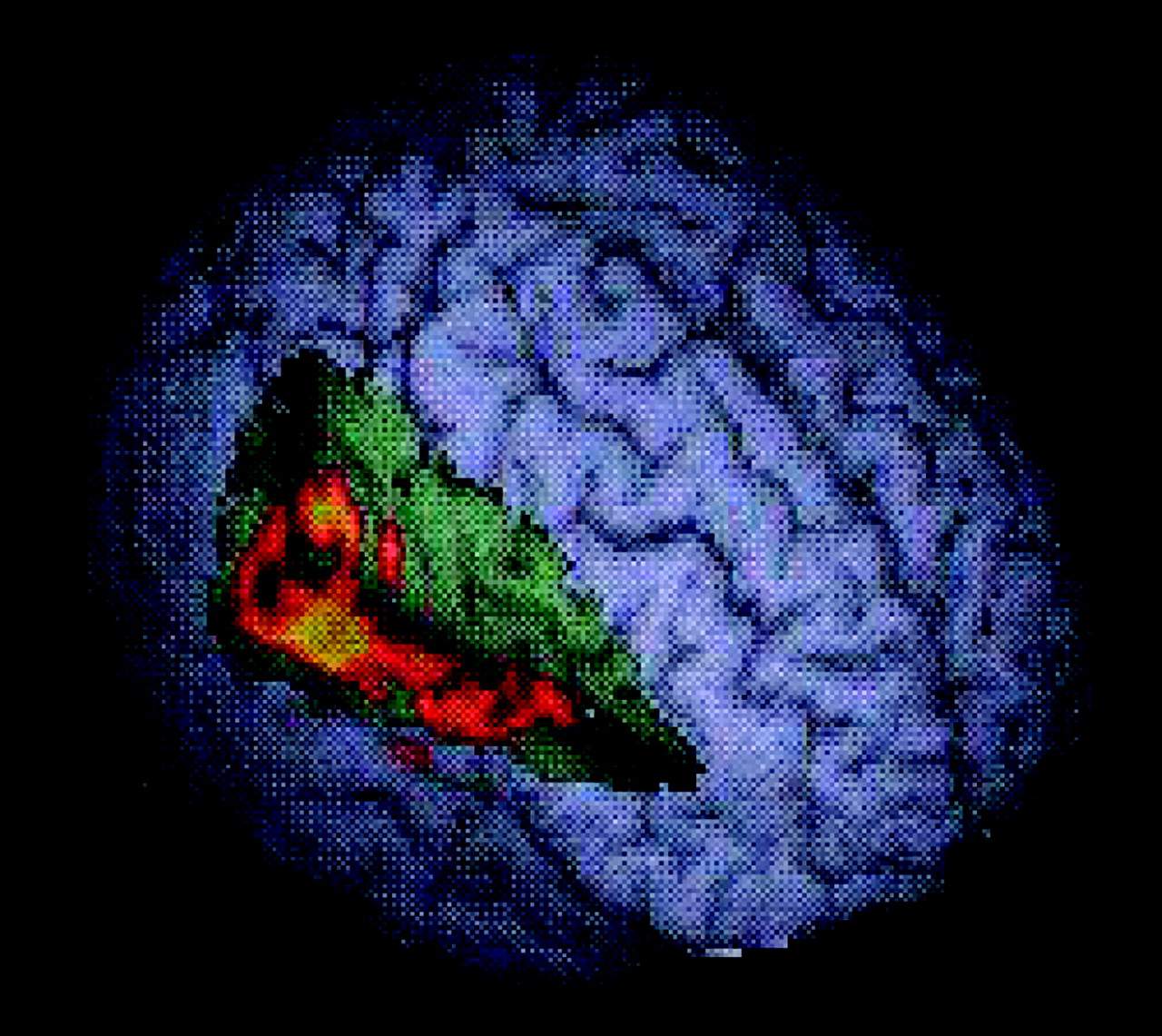
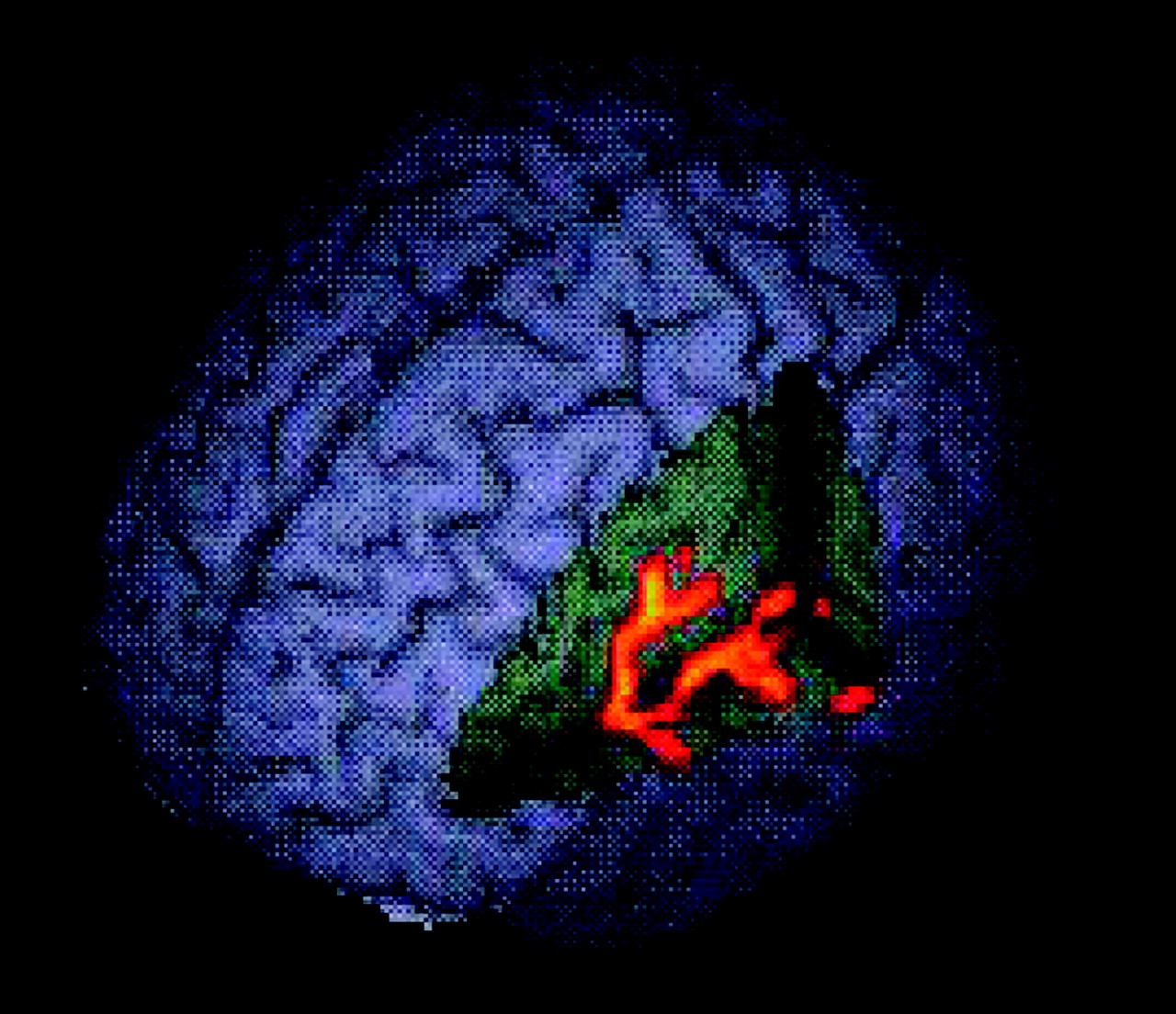
During switch trials, healthy subjects activated the right anterior cingulate, bilateral basal ganglia, and temporal and inferior parietal cortices. Patients with ADHD activated the right superior prefrontal cortex, bilateral anterior cingulate, and left inferior parietal lobe. Group comparison revealed two clusters of increased activation in healthy subjects compared with patients, one extending from the left inferior prefrontal to superior temporal gyrus and insula (
Figure 4 ) and another more right-sided cluster that extended from the inferior prefrontal gyrus through superior and middle temporal to inferior parietal cortex (
Figure 5 ).
Group Differences in Heterogeneity of Activation
Greater numbers of healthy subjects versus patients (27 versus 14) in the switch comparison may have influenced group differences in activation: if the variance in brain activation is comparable, then the difference in N is not problematic. If the variance is greater in the smaller group, then tests of differences may be too liberal; conversely, if variance is greater in the larger group, then tests of difference may be conservative. Coordinates (x, y, z) were extracted for both clusters of differences in activation for each subject. A principal component analysis was performed on the standardized residuals (to control for group membership bias) using an unrotated factor solution based on the correlation matrix. One principal component for each cluster of difference in brain activation emerged, explaining between 55% and 59% of the variances. Increased variance was observed for the first principal component of the residuals of the peak coordinates of the left hemisphere cluster of activation difference in the larger group of healthy subjects (F=4.299, df=39, p=0.045), suggesting that this comparison is conservative. No group differences in variance were observed in the right hemisphere cluster.
Covarying for Age
Because the age difference between the two groups approached significance, we computed correlations between the strength of significant clusters in the ANOVA analysis and age, although none were significant: go/no go task (r=0.003, p=0.988); switch task (left hemisphere cluster: r=0.18, p=0.27; right hemisphere cluster: r=0.30, p=0.06). However, given the potential impact of age and its borderline significant relationship with the right hemisphere cluster of increased activation in healthy subjects during the switch task, age was included as a covariate in ANCOVAs on imaging and performance data. All clusters showing between-group differences of activation within the go/no go and the switch tasks remained significant. For performance data, entering age as a covariate resulted in the between-group differences becoming nonsignificant for variability of reaction time for the go/no go (F=2.2, df=1, 32, p=0.15), motor Stroop (F=3.4, df=1, 40, p=0.07), and switch (F=3.9, df=1, 38, p=0.06) tasks. The significant difference in mean reaction time to repeat trials during the switch task also disappeared (F=2.4, df=1, 38, p=0.13).
Controlling for Conduct Disorder
To control for the potentially confounding effect of comorbid conduct disorder, we reanalyzed the group comparison removing those subjects with comorbid conduct disorder (go/no go: N=13; switch: N=11). All original significant clusters of between-group differences remained significant.
Discussion
Despite similar task performance, medication-naive boys with ADHD showed hypoactivation in task-specific brain regions compared with healthy subjects in two tasks: reduced activation in rostral mesial prefrontal cortex during the go/no go task and in right hemispheric inferior prefrontal, temporal, and parietal brain regions during the switch task. No differences in brain activation were observed during the motor Stroop task.
Patients were not impaired on the specific performance measures in this study: we wanted to equalize performance across the two groups, and so tasks were easier and slower versions of the same tasks in which, except for the Stroop task, we have found performance deficits in children and adolescents with ADHD
(32) .
Deficits in the rostral mesial frontal cortex in ADHD subjects during motor response inhibition support previous functional imaging findings reporting mesial prefrontal underactivation in ADHD during a stop task
(10), a go/no go task
(9), and a motor timing task
(10) . The reduced rostral prefrontal activation during intact inhibitory performance may be related to comeasured processes of selective attention and decision making
(8,
20), since more lateral prefrontal brain regions such as right dorsolateral and inferior prefrontal cortices are thought to mediate inhibitory control
(20) . Rostral mesial prefrontal cortex activation in adults during go/no go task performance has been related to decision making/response selection aspects of the inhibitory processes measured in this task
(20,
33) . Reduced activation in the mesial prefrontal cortex has been observed in ADHD during go/no go
(9), stop
(10), Stroop
(34), and timing tasks
(10) . Our study shows for the first time that this region is also underactivated in medication-naive children and adolescents with the disorder, suggesting that difficulties in recruitment of the mesial prefrontal cortex are inherent to ADHD pathology and unconfounded by medication history.
Performance of healthy adults on the same switching task used in this study has been shown to be mediated by prefrontal, temporal, and parietal cortices, regions presumably concerned with inhibition of irrelevant stimulus-response associations (prefrontal lobe), preparation of potential action (temporal lobe), and facilitating sustained visual attention (parietal lobe)
(21) . Right inferior prefrontal activation has been strongly associated with inhibitory processes in stop
(8,
33), go/no go
(20,
33), and switch
(20,
21) tasks and has emerged in adults as the common substrate for a shared inhibitory process in a conjunction analysis across tasks of switching and motor inhibition
(35) . We thus speculate that underactivation of the right inferior prefrontal cortex in ADHD during the switch task may be attributable to inhibitory processes. This is supported by the finding of underactivation of the right inferior prefrontal cortex during a stop task in this same group of medication-naive children and adolescents with ADHD
(11) and in an earlier sample of boys with ADHD
(10) . Underactivation of the right inferior prefrontal cortex in the same patient group during both successful stop and switch task performance suggests an underfunctioning neural substrate for an inhibitory mechanism required for both of these tasks.
The finding of right fronto-temporo-parietal hypoactivation in ADHD extends the deficit beyond hypothesized impairment of frontostriatal networks. While predominantly frontal lobe tasks elicit frontal abnormalities, the switch task, with its relatively greater load on parieto-temporal activation
(21), gives rise to additional deficits in these areas in patients with ADHD. This study supports structural and metabolic findings of abnormalities in temporal and parietal as well as prefrontal cortices: structural MRI studies found reduced bilateral parietal lobe volumes
(36) and reduced lateral-temporal and parietal cortex surfaces in children with ADHD
(37) . Functional rest studies using SPECT found reduced blood flow in right middle temporal and prefrontal cortex
(38) and left frontal and parietal regions
(39) in children with ADHD. Blood flow in temporal cortex is shown to be inversely correlated to the degree of motor impairment in children with ADHD
(40) . Medial temporal
(41) and parietal lobes
(42) do not achieve maturity until at least age 10, which may be further delayed in children and adolescents with ADHD
(43) .
In this study, underactivation in mesial and fronto-parieto-temporal brain regions during go/no go and switch task performance, respectively, did not lead to impaired performance. Brain activation measures may be more sensitive to abnormalities than performance. Alternatively, ADHD patients might have used idiosyncratic, alternative compensatory brain activation patterns that did not survive the critical thresholds for the group activation contrasts.
The integrity of neural networks during motor Stroop task performance in boys with ADHD reflects controversial behavioral findings on the sensitivity of the Stroop task in ADHD
(19) and our findings of no impairment in performance scores in children with ADHD using an offline version of this task
(32) . However, an fMRI study of adults with ADHD found hypoactivation of the anterior cingulate during a counting Stroop task
(34), which may have been more challenging than our motor Stroop task. Alternatively, the neural activation of adult patients may differ from that of children and adolescents with ADHD or may also have been altered by their long-term exposure to stimulant medication.
A few ADHD cases could not be scanned, which resulted in varying Ns for each task. This may have introduced a bias and limits the generalizability of findings and also the generalizability concerning medication-naivety across tasks. However, this study is a first attempt to investigate neurofunctional abnormalities in ADHD independent of potential confounds of chronic stimulant medication. Comparisons of ADHD patients with and without medication history within the same study design are needed to further clarify the extent and nature of the effects of long-term medication.
In conclusion, this study shows that medication-naive children and adolescents with ADHD demonstrate task-specific underactivation during performance of motor response inhibition and task switching that is not limited to the prefrontal cortex but also includes parietal and temporal cortices.