A dysfunction in neural connectivity has long been proposed as the fundamental abnormality underlying schizophrenia
(1 –
3) . Given that white matter constitutes the anatomical infrastructure for neural connectivity, it is reasonable to hypothesize the existence of white matter abnormalities in patients with schizophrenia
(4) . The present study aimed to investigate for white matter abnormalities in patients with schizophrenia, both at the time of their first presentation to mental health services with psychotic symptoms and longitudinally over the first 2 to 3 years of illness.
Volumetric white matter reductions, particularly in the frontal lobe, have been the most consistently reported white matter abnormality in patients with chronic schizophrenia
(5) . This volumetric reduction could potentially be indicative of abnormal axonal myelination, or it could represent axonal elimination resulting from neuronal death. In contrast to chronically ill patients, however, few studies have investigated white matter abnormalities in patients experiencing their first episode of schizophrenia. This is an issue that must be addressed, since investigating the structural underpinnings of schizophrenia early in its course gives significant insight into the nature of the disease, its origins, its clinical course, and the optimal path for therapeutic intervention. Furthermore, investigating patients with first-episode schizophrenia minimizes the confounds associated with long-term exposure to neuroleptic medication, which has been suggested to affect brain structure in and of itself
(6) . Previous studies that have investigated for evidence of white matter irregularities in first-episode schizophrenia patients have produced equivocal results. For example, while several studies have reported white matter abnormalities in first-episode schizophrenia, including impaired myelination of the corpus callosum
(7), irregular shape of the corpus callosum
(8), reduced fractional anisotropy in frontotemporal white matter
(9), and a decreased magnetization transfer ratio in the fasciculus uncinatus
(10), others have failed to observe any white matter abnormalities
(11 –
13) . Inconsistent results have also been reported in the very few studies that have investigated longitudinal white matter changes in patients with recent-onset schizophrenia. For example, while Ho et al.
(14) reported progressive atrophy in frontal lobe white matter over 3 years in patients with recent-onset schizophrenia, Rapoport et al.
(15) did not observe differential rates of white matter change over 4 years between patients with childhood-onset schizophrenia and matched healthy comparison subjects.
Aside from the clearly delineated corpus callosum, it is notoriously difficult to manually define white matter regions of interest consistently between subjects because of the dearth of referential anatomical landmarks. Hence, the majority of previous studies have investigated changes in white matter at the level of the whole brain or the brain lobe. The lack of sensitivity inherent in such a large-scale analysis could well have contributed to the inconsistency of previous findings. This is one area in which automated statistical imaging techniques (e.g., voxel-based morphometry) are advantageous. By looking for evidence of structural difference at every voxel in the brain, the statistical imaging techniques are able (given appropriate statistical correction for multiple comparisons) to identify small, discrete areas of regional abnormality without requiring the manual tracing of regions of interest which, as well as being difficult to define consistently, are also necessarily constrained to the regions defined by prior hypotheses.
In this study, we used voxel-based morphometry to investigate for evidence of white matter abnormality in patients with first-episode schizophrenia relative to matched healthy comparison subjects (baseline condition). We then used tensor-based morphometry to identify evidence of progressive white matter atrophy in the first-episode schizophrenia patients over the first 2 to 3 years of illness, over and above any corresponding longitudinal changes experienced by the healthy comparison subjects (follow-up condition). Based on the longitudinal gray matter reductions that we have previously reported in these patients
(16), we hypothesized that the first-episode schizophrenia patients would exhibit frontal, temporal, and parietal white matter reductions at baseline relative to the healthy comparison subjects and that these regional abnormalities would degenerate over the follow-up interval.
Discussion
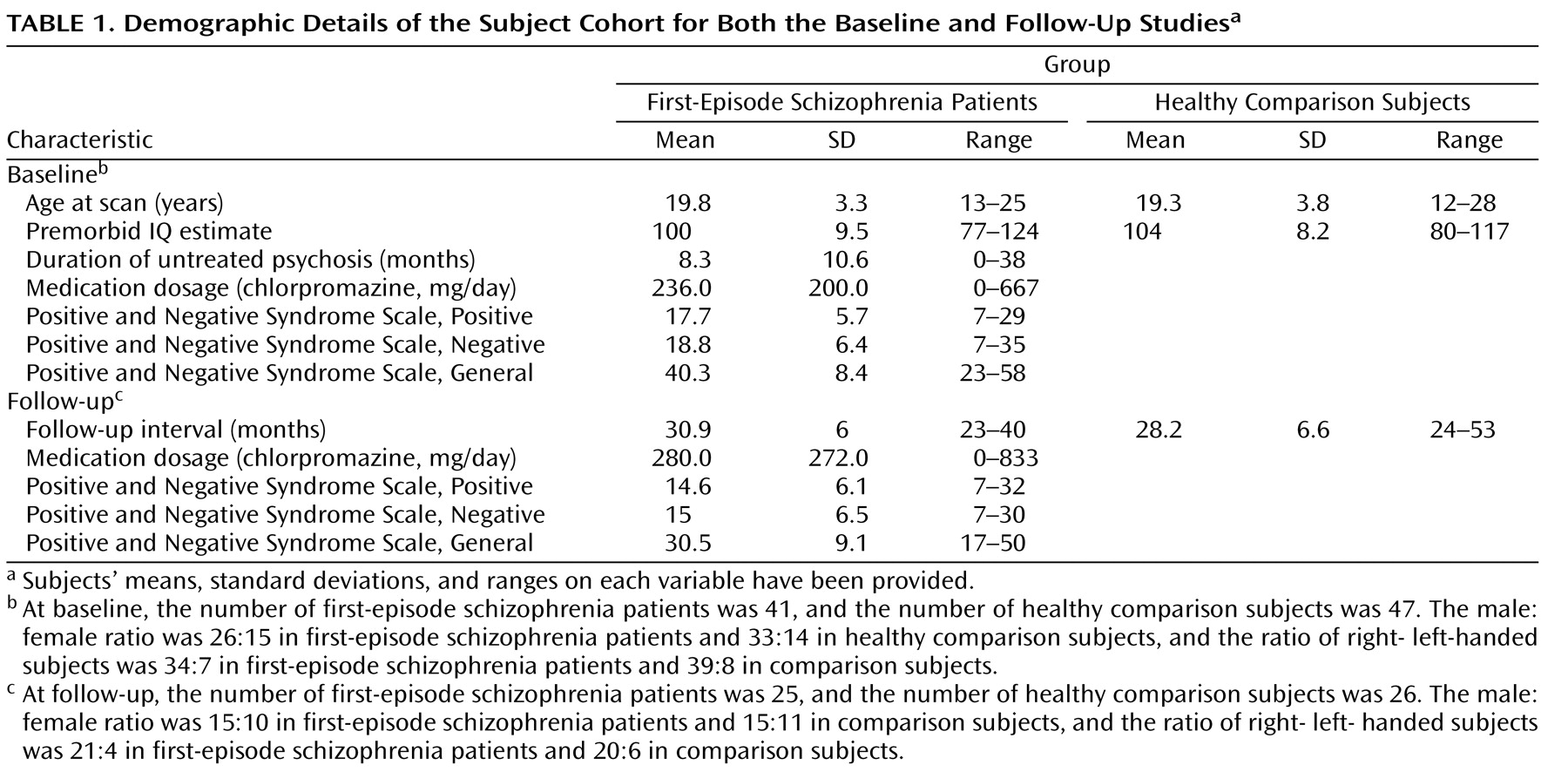
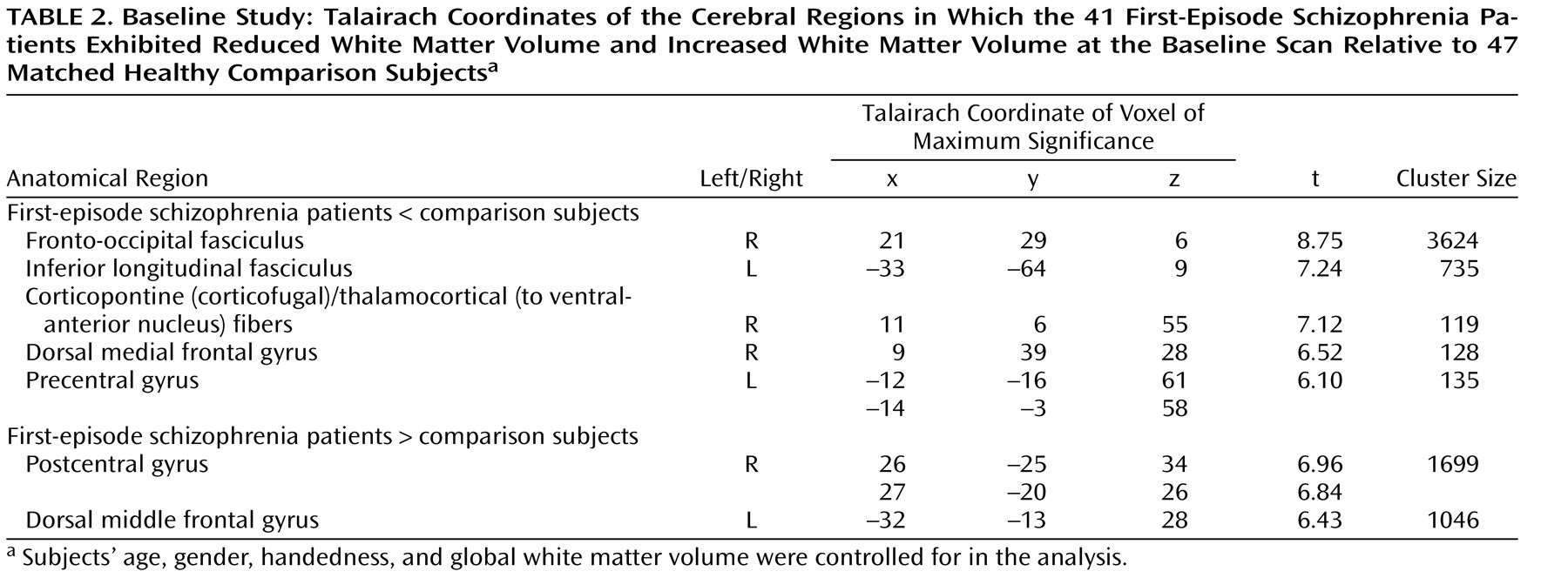
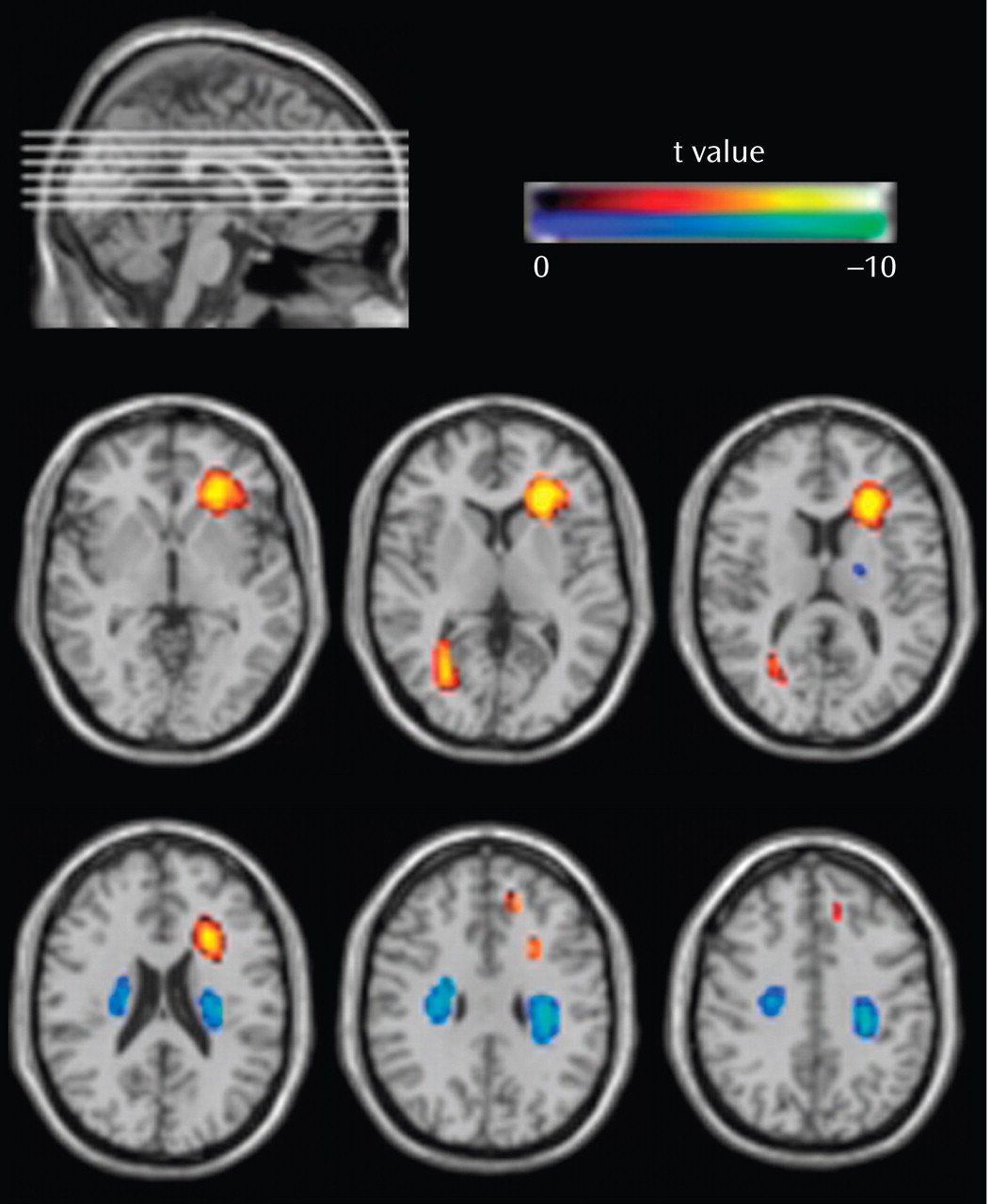
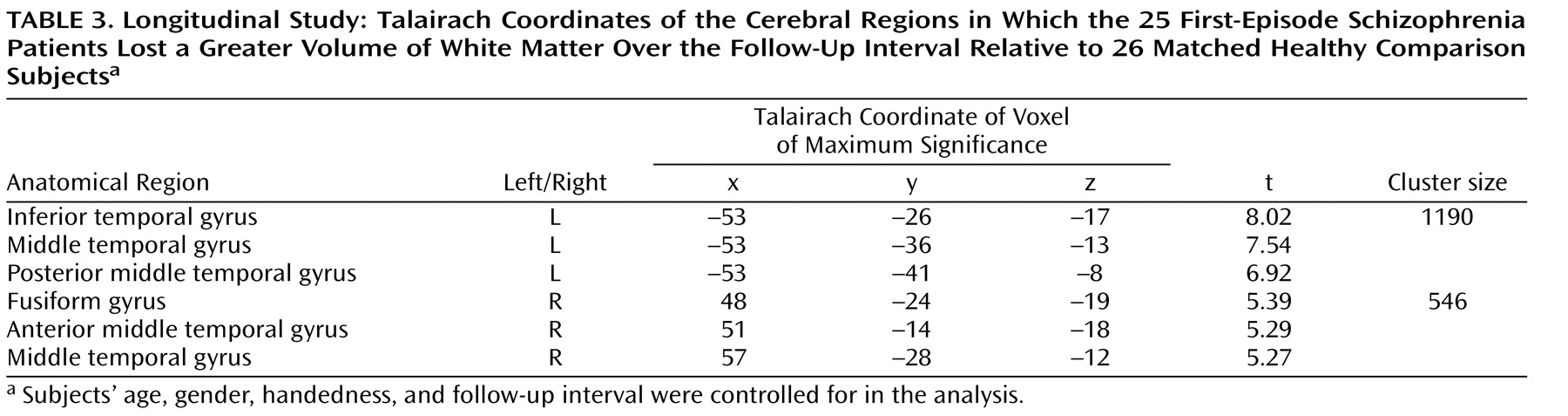
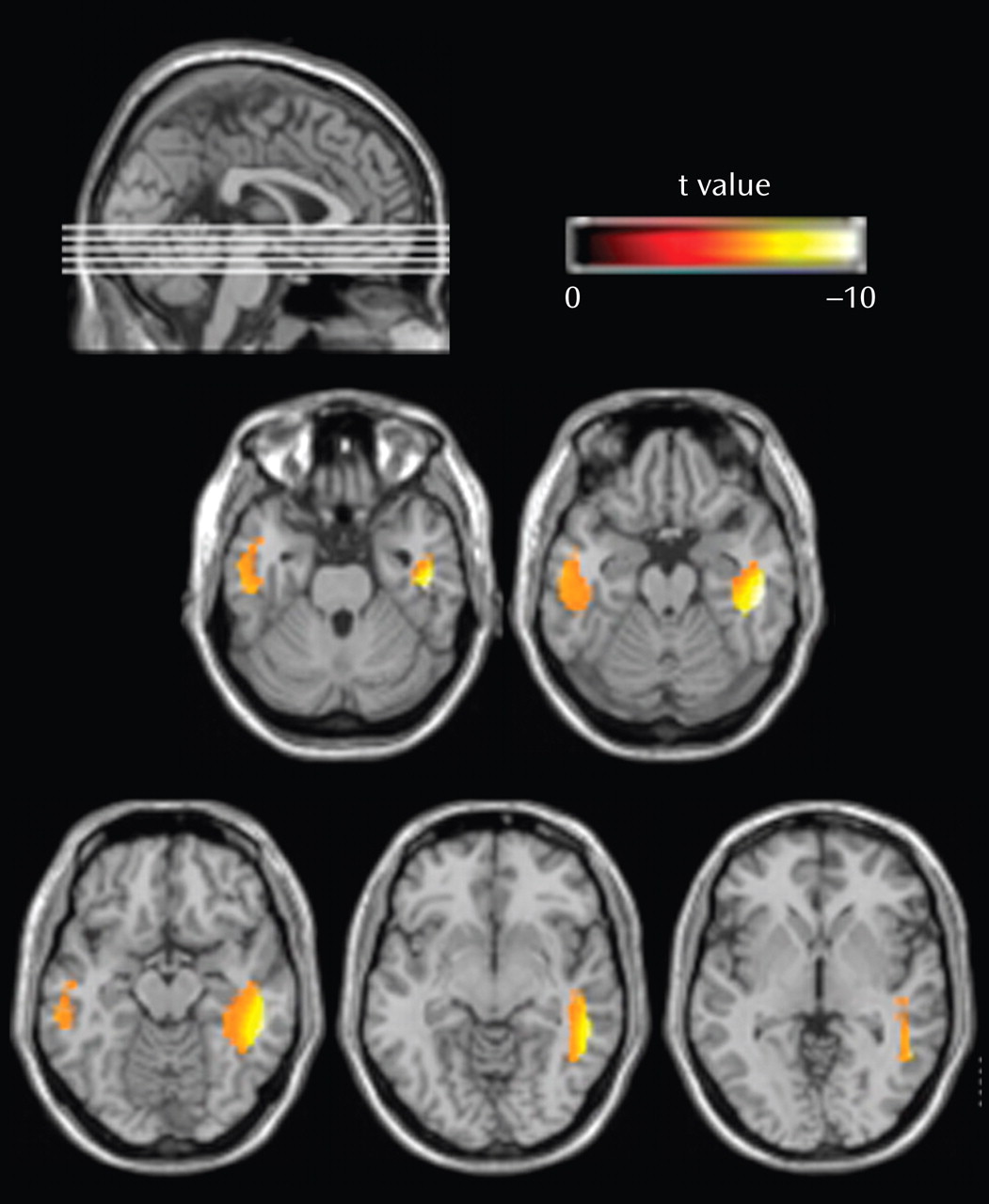
In spite of the fact that schizophrenia has long been thought of as a disorder of brain connectivity, few studies have investigated for evidence of white matter abnormalities in patients with the disease, and almost none have examined whether white matter pathology is static or progressive over time. In this study, we used voxel-based morphometry to identify volumetric white matter reductions in the frontal and temporal lobes and white matter increases at the frontoparietal junction bilaterally in 41 first-episode schizophrenia patients relative to 47 matched healthy comparison subjects (baseline study). Furthermore, we also used tensor-based morphometry to identify abnormal progressive volumetric white matter reductions in the temporal cortex bilaterally over the first 2 to 3 years of illness in 25 first-episode schizophrenia patients relative to 26 matched comparison subjects (follow-up study). These results suggest that white matter abnormalities are present in patients with schizophrenia at the time of their first presentation to mental health services with psychotic symptoms and that these abnormalities progress over at least the first few years of illness.
The baseline results are consistent with a number of recent studies that have inferred the existence of white matter abnormalities in patients with first-episode schizophrenia. Szeszko et al.
(9), for example, used diffusion tensor imaging and found that first-episode schizophrenia patients exhibited reduced fractional anisotropy, which has been shown to be reduced in patients with the demyelinating disease multiple sclerosis
(29), in the internal capsule and in the white matter of the middle frontal gyrus and superior temporal gyrus. Similar results were reported by Hao et al.
(30), who observed that first-episode schizophrenia patients exhibited globally reduced fractional anisotropy relative to comparison subjects, which was most significant in the white matter of the inferior frontal gyrus, cerebral peduncle, and orbitofrontal cerebrum. Furthermore, Bagary et al.
(10) used magnetization transfer imaging and observed abnormal hypointensities (which is a putative measure of demyelination) in patients with first-episode schizophrenia in the white matter of the prefrontal cortex, insula, and fasciculus uncinatus.
In contrast, while a number of studies have reported volumetric white matter abnormalities in patients with chronic schizophrenia
(31,
32), to our knowledge, this is the first study to report volumetric white matter abnormalities in patients with first-episode schizophrenia. The majority of previous volumetric studies have investigated patients’ white matter volumes at either the level of the whole brain or the level of the brain lobe, and they have failed to observe any significant volumetric abnormalities in patients with first-episode schizophrenia
(11 –
13) . Thus, it appears as though the white matter abnormalities present in patients with first-episode schizophrenia are subtle and limited in extent and possibly only detectable via a voxel-based analysis.
In addition to the baseline white matter abnormalities, the first-episode schizophrenia patients also exhibited abnormal, progressive white matter atrophy in the inferior temporal lobe over the first 2 to 3 years of their illness. While there have been several studies in the literature that have reported longitudinal gray matter atrophy over the first few years of illness in patients with first-episode schizophrenia
(33,
34), there has been (to the best of our knowledge) only one previous study that has reported longitudinal white matter change over this period. Ho et al.
(14) used an automated masking procedure to segment patients’ MRI into the frontal, temporal, and parietal lobes. They found that patients with recent-onset schizophrenia lost a greater amount of frontal-lobe white matter over a 3-year interval relative to a group of matched healthy comparison subjects, who actually gained white matter over this period. Thus, the results of our study, when considered in combination with the findings of Ho et al.
(14), support the possibility of progressive frontotemporal white matter atrophy in patients with first-episode schizophrenia over at least the first few years of their illness.
Given the role that white matter plays in connecting disparate regions of neural tissue and in modulating the transmission velocities of action potentials, volumetric white matter abnormalities (be they volumetric increases or decreases), as we have reported in the present study, could be expected to result in a dysfunction in neural communication and a disintegration of neural activity
(4) . Such disintegration in neural activity has previously been argued to underlie the cognitive disorganization that is characteristic of schizophrenia
(1,
4) . Furthermore, others have argued that certain specific symptoms of psychosis (such as delusions of control) could arise from disintegration between the neural correlates of actions and the neural correlates of perceiving their intended consequences
(35) . The fact that we observed the white matter of the frontal and temporal lobes to be especially affected in patients with first-episode schizophrenia adds support to those theories that have argued for a breakdown in frontotemporal connectivity as being the underlying cause of schizophrenia
(3,
36,
37) .
In a previous study using the same participant cohort, we observed evidence of substantial abnormal gray matter atrophy in the first-episode schizophrenia patients over the 2- to 3-year follow-up interval. This raised the question of whether the white matter abnormalities reported in the present study were secondary to these gray matter changes. We suggest that this scenario is unlikely for two reasons. First, the regions of longitudinal white matter abnormality in the first-episode schizophrenia patients remained when we controlled for the degree of subjects’ gray matter loss over the follow-up interval. Second, relative to the widespread gray matter atrophy exhibited by the first-episode schizophrenia patients over the follow-up interval (which engulfed most of the parietal cortex), there was comparatively little white matter atrophy over the same period, and it was confined to the inferior temporal lobe. Given that oligodendrocytes (which constitute the bulk of the white matter) depend on their corresponding neuronal axons for survival
(38), the fact that we observed widespread progressive gray matter loss but relatively circumscribed white matter loss suggests that the first-episode schizophrenia patients did not experience widespread neuronal death over the follow-up interval. This suggestion is consistent with previous stereological studies that have failed to observe a reduction in neuron number in the neocortex of patients with schizophrenia
(39) . Instead, the results of the present study are consistent with the “reduced neuropil hypothesis”
(40), which argues that the characteristic gray matter loss exhibited by patients with schizophrenia is underpinned by a reduction in the numbers of dendrites, dendritic spines, and glial cells (all of which can occur without a corresponding reduction in white matter), rather than the death of neuronal cell bodies and their associated axons.
In summary, this study is the first (to our knowledge) to report volumetric white matter abnormalities in patients with first-episode schizophrenia. Furthermore, we also observed evidence of abnormal, progressive white matter atrophy over the first 2 to 3 years of illness in these patients. Given the role that white matter plays in neural communication, we suggest that these white matter abnormalities may underlie the dysfunctional neural connectivity that has been proposed as being a fundamental cause of schizophrenia.