The importance of craving experiences in perpetuating human drug addiction has been frequently asserted
(1–
3). Drug craving is thought to be a powerful motivational state or intense desire that drives the cocaine user to seek cocaine. However, the specific psychological mechanisms, affective and cognitive, that underlie drug craving, its determinants, and its relationship to subsequent drug taking are not fully understood. Phenomenologically, cocaine users report that craving occurs roughly twice per day (with each episode lasting approximately 20 minutes or less
[4]), is of variable intensity, and can be induced by multiple means. For example, cocaine administration can reinstate responding for cocaine in rats
(5) and has been demonstrated to induce craving in humans for additional cocaine
(6). Laboratory-based techniques that induce stress have recently been shown to evoke increased self-reported craving in cocaine abusers
(7). A corpus of research also suggests that drug-related environmental cues can serve to elicit craving in drug users
(3,
8,
9) and that the strength of such cue-induced craving is positively correlated with the severity of cocaine dependence
(10). Childress and colleagues
(8) reported that cocaine users frequently cite as craving initiators external cues such as money or a drug-using friend and internal cues such as dysphoria. An analysis of the determinants of crack cocaine relapse noted that 34% of relapses followed encounters with drug-related stimuli, and 11% followed money handling
(11). However, the neuronal sites and psychological systems responsible for initiating and maintaining cue-induced cocaine craving and how they may differ from other arousal states are not well understood. Such data would appear critical for the development of new behavioral and pharmacological interventions for cocaine treatment, a situation made more urgent as current therapeutic regimens are less than totally effective for the majority of individuals who seek treatment. Noninvasive neuroimaging techniques now afford the opportunity to identify the neuroanatomical underpinnings of these psychological phenomena.
Previous neuroimaging research has implicated a number of cortical and subcortical regions in human drug craving, including prefrontal and limbic structures. For example, significant correlations between self-reported craving scores and regional cerebral glucose metabolism in prefrontal and orbitofrontal cortex have been reported
(12). A similar frontal involvement was observed by Maas and colleagues
(13), who by means of functional magnetic resonance imaging (fMRI) reported significant activation in the left dorsolateral prefrontal cortex and anterior cingulate that corresponded with presentation of cocaine-related stimuli. It has also been reported that cocaine cues, relative to neutral cues, produced increases in regional cerebral glucose metabolism in the dorsolateral prefrontal, medial orbitofrontal, retrosplenial, peristriate, and a number of temporal and parietal regions
(14). Significant correlations of 0.60 or greater were found between self-reported craving measures and regional cerebral glucose metabolism in the dorsolateral prefrontal cortex, cerebellum, and medial temporal lobe, specifically the amygdala. Other neuroimaging studies have replicated a frontal and limbic involvement in cocaine craving
(15–
17). It might be conjectured that the emerging picture of widespread neuronal involvement reflects the participation of a number of cognitive and emotional processes working in concert to produce the subjective craving experience.
The determination that craving has been induced through exposure to cocaine-related cues requires that certain criteria be met. It has been proposed that the craving response should be both population and content specific, being observed in cocaine users but not in cocaine-naive comparison subjects and in response to cocaine stimuli but not to, say, opiate-related cues
(18). We suggest that the appropriateness of the content-specific criterion be contingent upon one’s choice of comparison stimuli. For example, an increase in arousal may be a component of cue-induced cocaine craving. While it may be reasonable to require that this arousal not be induced by otherwise neutral stimuli (e.g., opiate-specific paraphernalia, as demonstrated by Ehrman et al.
[19]), one might expect a similar response to other arousing stimuli (e.g., sexually evocative stimuli). Indeed, the degree to which the neuroanatomical response to cocaine cues is mirrored in response to other evocative stimuli is an open question that may illuminate some of the (common) processes evoked by both sets of cues. To this end, the present study sought to determine if a neuroanatomical response observed in cocaine users when exposed to cocaine-related stimuli would be unique to cocaine users (i.e., not present in cocaine-naive comparison subjects) and unique to the cocaine stimuli (i.e., not present for neutral stimuli) but perhaps shared by nondrug evocative stimuli.
Method
Subjects
In total, 24 experienced cocaine users and 18 healthy comparison subjects participated in this study. The subjects were recruited through local newspaper advertisements and were paid for their participation. The cocaine users were screened so that only those whose primary method of cocaine administration was through freebase (crack) smoking were included. No subject met criteria for any axis I psychiatric condition other than cocaine abuse or dependence. Seventeen cocaine users (14 men and three women; mean age=34 years, age range=27–44) and 14 comparison subjects (nine men and five women; mean age=26 years, age range=19–39) passed all exclusionary criteria and were included in the fMRI analyses. Of the 17 cocaine users, nine were Caucasian, and eight were African American; 13 were strongly right-handed, one was left-handed, and three were ambidextrous. Of the 14 comparison subjects, 12 were Caucasian, one was Asian, and one was Hispanic; 13 were strongly right-handed, and one was left-handed. The cocaine users averaged 11 years of cocaine use (range=2–25) with an average monthly cocaine expenditure of $1,025 (range=$150–$5,000). The comparison subjects reported no history of cocaine use. After complete description, all subjects gave written informed consent to participate in this study, which was approved by the institutional review board of the Medical College of Wisconsin.
Procedure
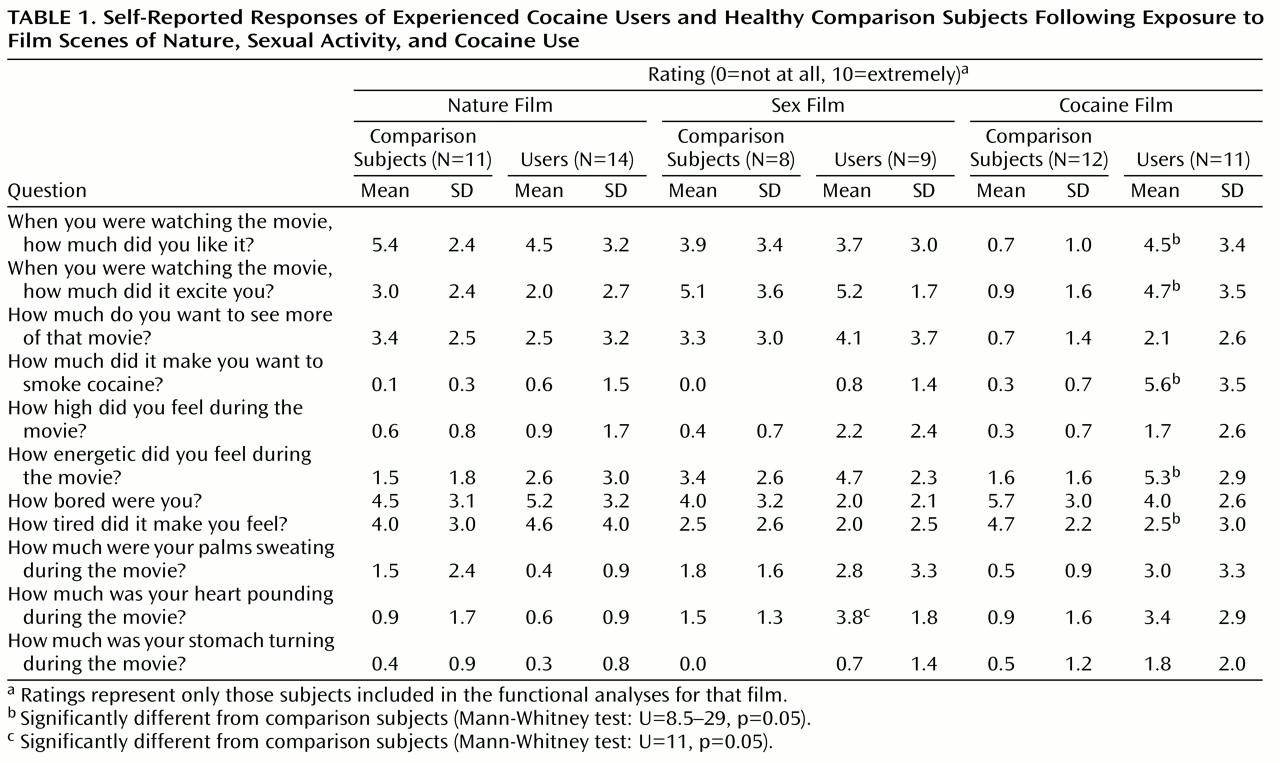
Upon arrival at the MRI unit, each subject completed consent forms and received instructions for, and practiced on, a working memory task that would be performed during the scanning session. Video film segments and the working memory task were back-projected onto a screen at the subject’s feet and were viewed with the aid of prism glasses attached to the inside of the radio frequency head coil. The video dialogue was delivered to the subjects by air conduction through plastic tubes threaded through earplugs that attenuated scanner noise. Three films with different content were used. The cocaine film depicted two African American men engaged in drug-specific dialogue while smoking “crack cocaine” (which was actually benzocaine made to look like crack cocaine) and drinking “alcohol” (which was water in a gin bottle). The men were experienced cocaine users; the film was made in consultation with, and reviewed by, a number of former and current cocaine users to ensure authenticity. The nature film contained scenic outdoor images; the sex film contained explicit group heterosexual activity. Each film was 4 minutes in duration, and each was preceded by a 3-minute blank blue screen. All subjects first saw the nature film, and the order of the sex and cocaine films was counterbalanced across subjects. Immediately after each film, subjects performed a visuospatial working memory task for 5 minutes. Thus, each scanning run consisted of a 3-minute rest period, a 4-minute film, and a 5-minute working memory task. After the working memory trials that followed each film, subjects completed retrospective self-report measures evaluating their response to the previous film’s content. Questions focused on the subject’s responses to the film (
Table 1). The working memory task served a two-fold purpose: as a distracter to minimize any cue-induced craving cross-talk between films and as a probe to determine the effects of craving on cognitive task performance and brain activation. Results of this portion of the experiment will be reported elsewhere.
We could not control for the role that expectations of obtaining either cocaine or sex after the study may have had on the subjects. All cocaine users received a brief therapeutic “talk-down” intervention after the scanning procedure and were not allowed to leave the hospital until the attending psychiatrist certified that they were no longer craving cocaine. In a previous study
(20), no additional drug use subsequent to participation in an intravenous cocaine experiment was reported, which suggests little transfer from a drug-related experimental context to the real-world context.
fMRI Scanning Procedures
Contiguous 7-mm sagittal slices covering the entire brain were collected by using a blipped gradient-echo, echo-planar pulse sequence (TE=40 msec; TR=6000 msec; field of view=24 cm; 64 × 64 matrix; in-plane resolution=3.75 × 3.75 mm). All scanning was conducted on a 1.5-T Signa scanner (GE Medical Systems, Milwaukee) equipped with a 30.5-cm internal diameter three-axis local gradient coil and an end-capped quadrature birdcage radio frequency head coil
(21). Foam padding was used to limit head movements within the coil. High-resolution radio frequency spoiled gradient recalled acquisition in the steady state anatomic images were acquired before functional imaging to allow subsequent anatomical localization of functional activation.
fMRI Analyses
All data processing was conducted with the software package AFNI version 2.2
(22). In-plane motion correction and edge detection algorithms were first applied to the functional data. Subjects whose fMRI time series for a film still had perceptible residual head movements as determined by cine viewing were excluded from the analysis for that film. The number of cocaine users and comparison subjects included in the functional analyses for each film are presented in
Table 1. In total, 47% of the cocaine users’ films and 57% of the comparison subjects’ films were analyzable. Exclusion of 40% of the cocaine users’ films and 35% of the comparison subjects’ films was due to movement, with the remaining exclusions attributable to technical problems in the data acquisition. All of the cocaine users included in the critical cocaine film analyses were current users, and none were receiving treatment.
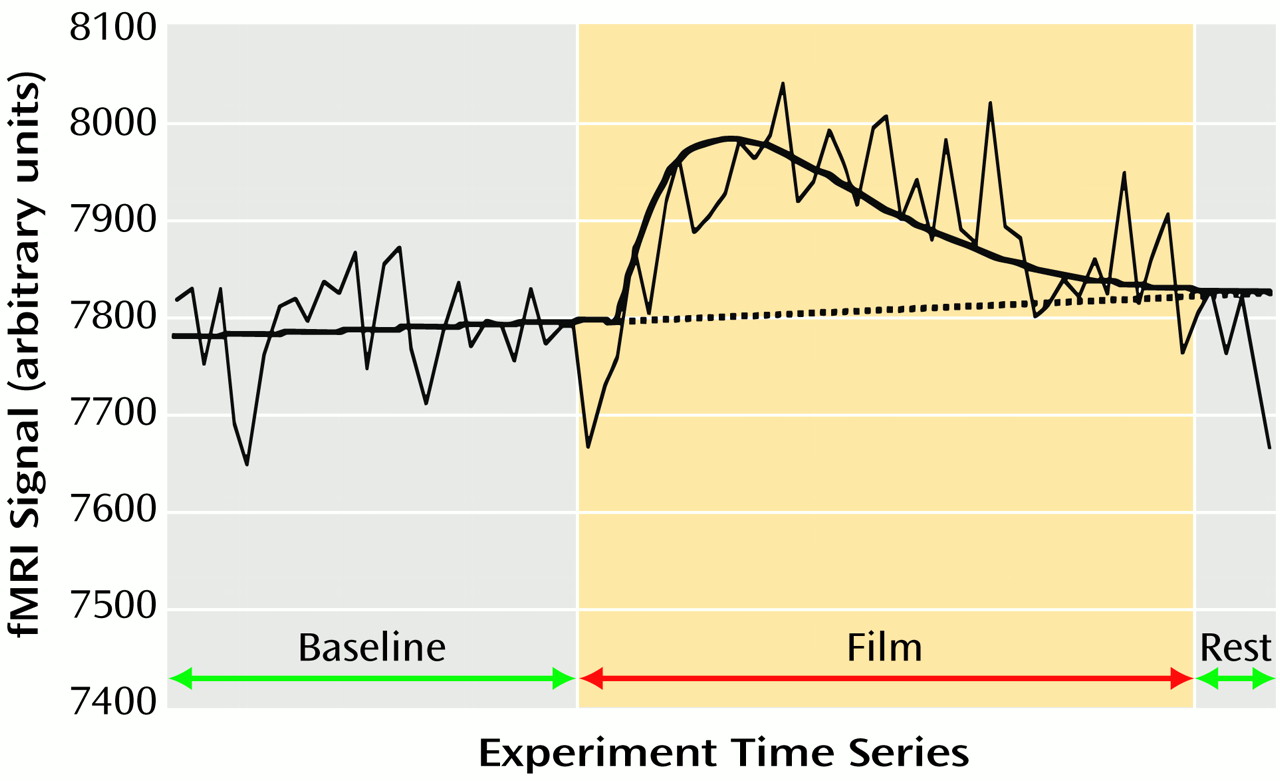
The first 7.5 minutes (75 images) of each scanning run were included in the present fMRI analysis. This included the 3-minute baseline period, the 4-minute film, and the first 30-second rest period of the subsequent working memory task. To characterize a voxel’s response, fMRI signals acquired over this 7.5-minute period were modeled with beta distributions on a per-voxel basis by using a nonlinear regression technique
(23) (
Figure 1). The beta distribution was chosen on empirical grounds, given the wide range of different time series that it can model. The onset time of the beta model was constrained to occur within 1.5 minutes after video onset, and the best linear fit was fitted to the time series before this onset time. The other parameters of the beta distribution (a multiplicative constant [k] and two exponents [α, β]) (
Figure 1) were loosely constrained in order that a best-fitting model for each voxel’s time series could be attained. The time series data were filtered to exclude all frequencies above 0.01 Hz before the nonlinear modeling, since preliminary analyses revealed that high-frequency changes in the data time series often adversely affected the goodness of the nonlinear model fit. For each voxel, the area under the curve of the beta model was expressed as a percentage of the area under the best linear fit (a null hypothesis representing no response). This percentage of the area under the curve measure served as an estimate of the magnitude of response of a given voxel to the film content (
Figure 1). The percentage of the area under the curve functional images were converted to a standard stereotaxic coordinate system
(24) and spatially blurred by using a 4.2-mm full width at half maximum isotropic gaussian filter. These functional images are referred to subsequently as activation maps and were used for the following group analyses.
Localization and Specificity of Cocaine Craving
A one-sample t test against the null hypothesis of no effect was performed on the percentage of the area under the curve measure for cocaine users viewing the cocaine film. This t test, thresholded with a voxel-wise p value of 0.0025 and a criterion that each significant voxel be part of a larger 100-μl cluster of contiguous significant voxels (roughly equal to the size of the originally acquired voxels), identified voxels that showed a response to the cocaine film in cocaine users. The advantages of combining a voxel-wise threshold with a minimum cluster size have been described elsewhere
(25). The clusters of activation that survived these criteria defined functional regions of interest for the following set of comparisons.
To determine if the response of these regions was unique to the cocaine users (population specificity), the activated regions of the cocaine users during exposure to the cocaine film were superimposed upon the activation maps of the comparison subjects during exposure to the cocaine film, and the regions of interest were compared by using two-sample t tests. To determine if the response of these regions of interest was specific to the cocaine film (content specificity), the activated regions of the cocaine users during exposure to the cocaine film were superimposed upon the activation maps of the cocaine users during exposure to the nature film, and t tests compared the mean activation values. Finally, to determine if these regions of interest were also activated by nondrug, evocative stimuli (content specificity), the activated regions of the cocaine users during exposure to the cocaine film were superimposed upon the activation maps of both the cocaine users and comparison subjects during exposure to the sex film. Separate t tests were performed for each comparison as the ad hoc nature of the exclusions from the functional analyses left too few subjects with complete data to allow a full factorial analysis of variance.
Responsivity to the Sex and Nature Films
To identify areas activated when viewing the sex film, separate one-sample t tests against the null hypothesis of no effect were performed on the activation maps of cocaine users and comparison subjects. Each was thresholded with a voxel-wise p value of 0.0025 and a 100-μl cluster criterion, as described earlier. To facilitate statistical testing between the two groups, these maps were combined so as to include a voxel if significant in either map. Two-sample t tests comparing users and comparison subjects were then performed on the mean activation values for each cluster of this combined map. An identical sequence of analyses were performed for the nature film.
Results
Self-Report Measures
Table 1 contains group averages of Likert scale responses to questions asked after each film. The data suggest that the cocaine film successfully induced a craving state in cocaine users. For example, the cocaine users reported liking the cocaine film more than comparison subjects while not differing from comparison subjects in how much they liked the nature or sex films. A similar pattern was observed for how excited and energized they were by the films and, critically, by how much each film made them want to smoke cocaine. Cocaine users also reported less tiredness than comparison subjects after the cocaine film only. To facilitate additional comparisons based on these responses to the cocaine film, a composite craving score was calculated. The five questions that significantly differentiated the cocaine users and the comparison subjects following exposure to the cocaine film but not the other two films were chosen as epitomizing cocaine craving. The composite craving score was the sum of these five questions (the tiredness measure was first subtracted from 10 such that less tiredness would be coded as increasing with increasing craving). The composite craving score allowed us to test if differences in cocaine craving existed between the excluded and included subjects and to test for effects of film order.
Composite craving scores significantly differed between all cocaine users (N=23; the data for one subject was lost) and comparison subjects (N=18) who viewed the cocaine film (t=6.7, df=39, p<0.0001) as well as between just those subjects who were included in the cocaine film functional analyses (t=6.4, df=21, p<0.0001). Further analyses revealed that cocaine users excluded from the cocaine film functional analyses due to head movements were not significantly different than the analyzed users in reported craving (t=1.9, df=17, p=0.07). In fact, subjects included in the functional analyses reported a higher composite craving score (27.7 versus 20.5), thus obviating concerns of a selection bias in our analyses whereby the subjects who craved most might have moved most. An analysis of the film order effect in cocaine users who viewed the cocaine film before or after the sex film revealed no differences in composite craving score. This was true for all cocaine users (t=0.4, df=21, p=0.73) and for just those included in the cocaine film functional analyses (t=0.1, df=9, p=0.91). A similar composite score was calculated for responses to the sex film (i. e., how much subjects liked the sex film, were energized by it, etc.) and to the nature film (i. e., how much subjects liked the nature film, were energized by it, etc.). No significant group differences were found with unpaired t tests for the sex or the nature film either when all subjects or just those subjects included in the respective functional film analyses were included.
Functional Activation Analyses: Cocaine Craving
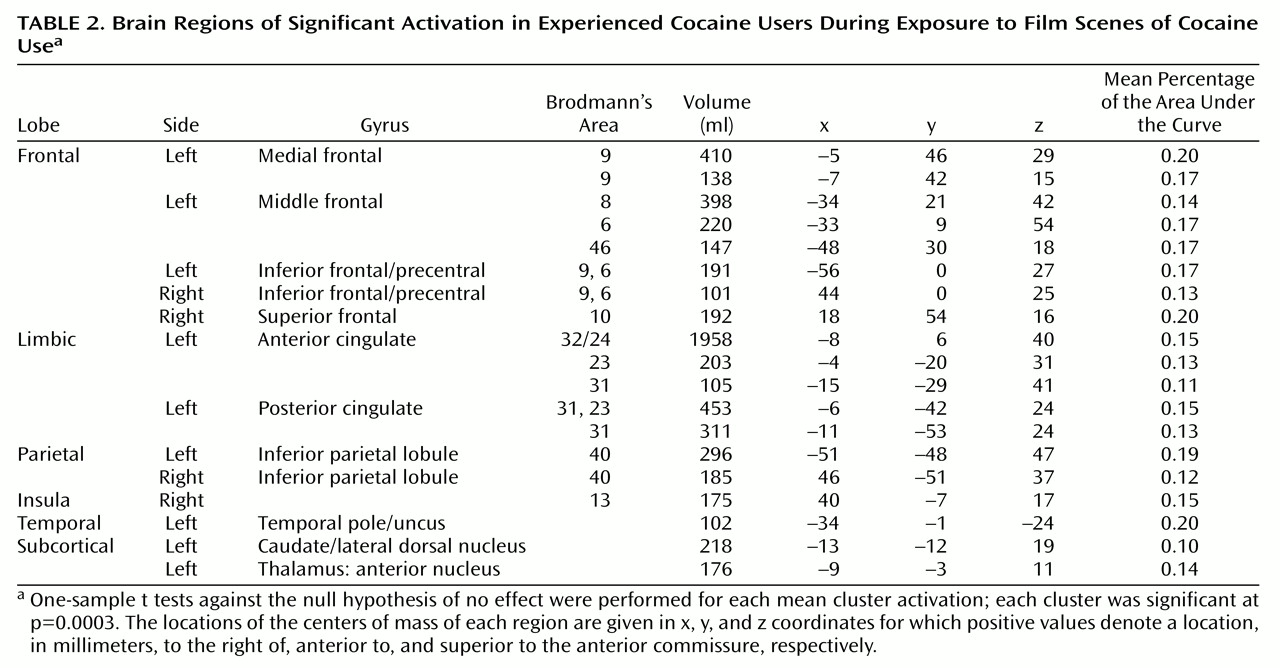
For the cocaine users, 19 regions of interest showed significant responses during exposure to the cocaine film (
Table 2). These were mostly in frontal and limbic lobes, generally left lateralized, and included the medial, inferior, middle, and superior frontal gyri as well as both the anterior and posterior cingulate gyrus. Bilateral activation was seen in the inferior parietal lobule, and left lateralized activation was seen in the temporal pole. The remaining regions of interest were observed in the right insula and subcortically, in the left caudate/lateral dorsal nucleus and the anterior nucleus of the thalamus.
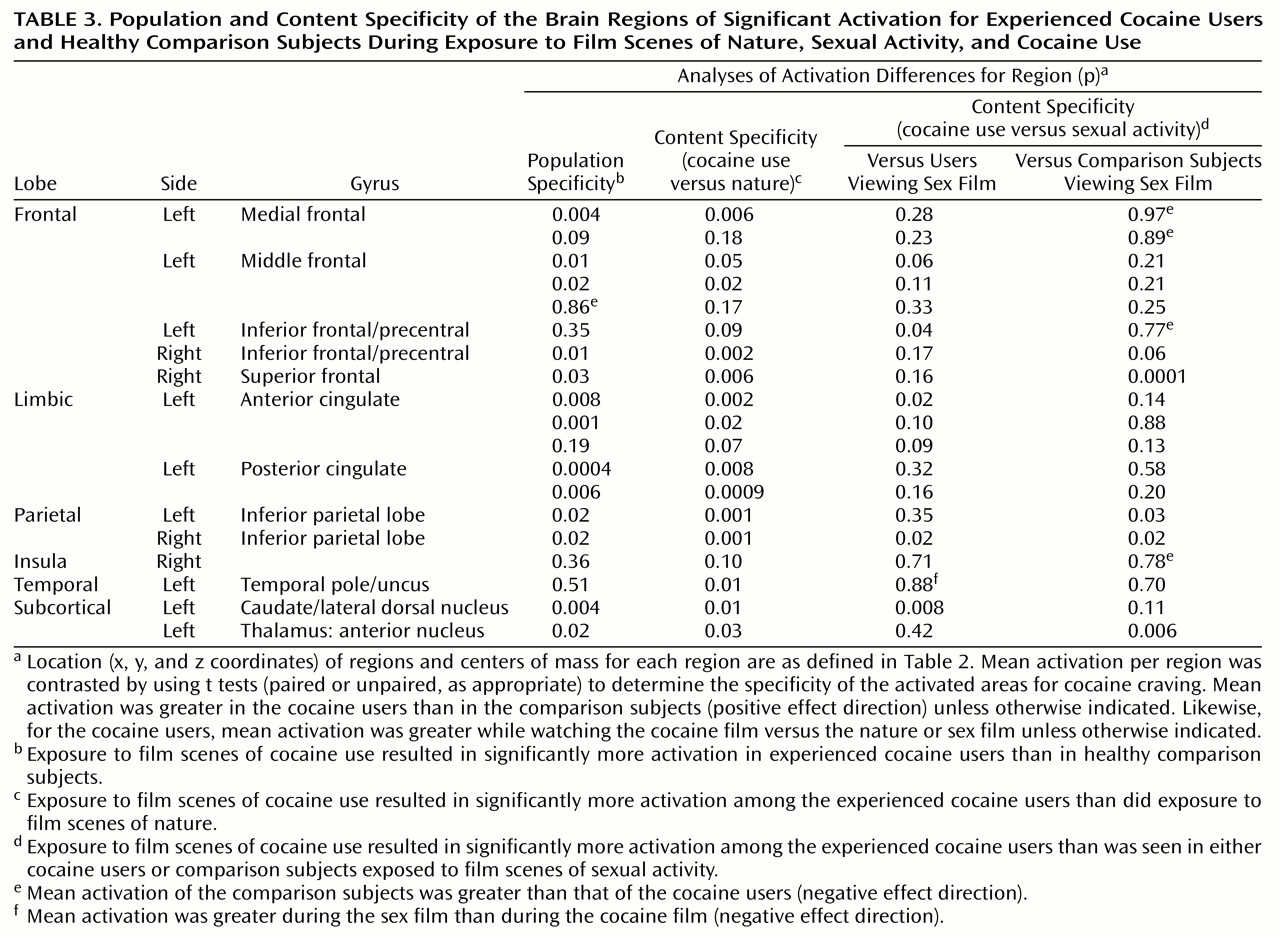
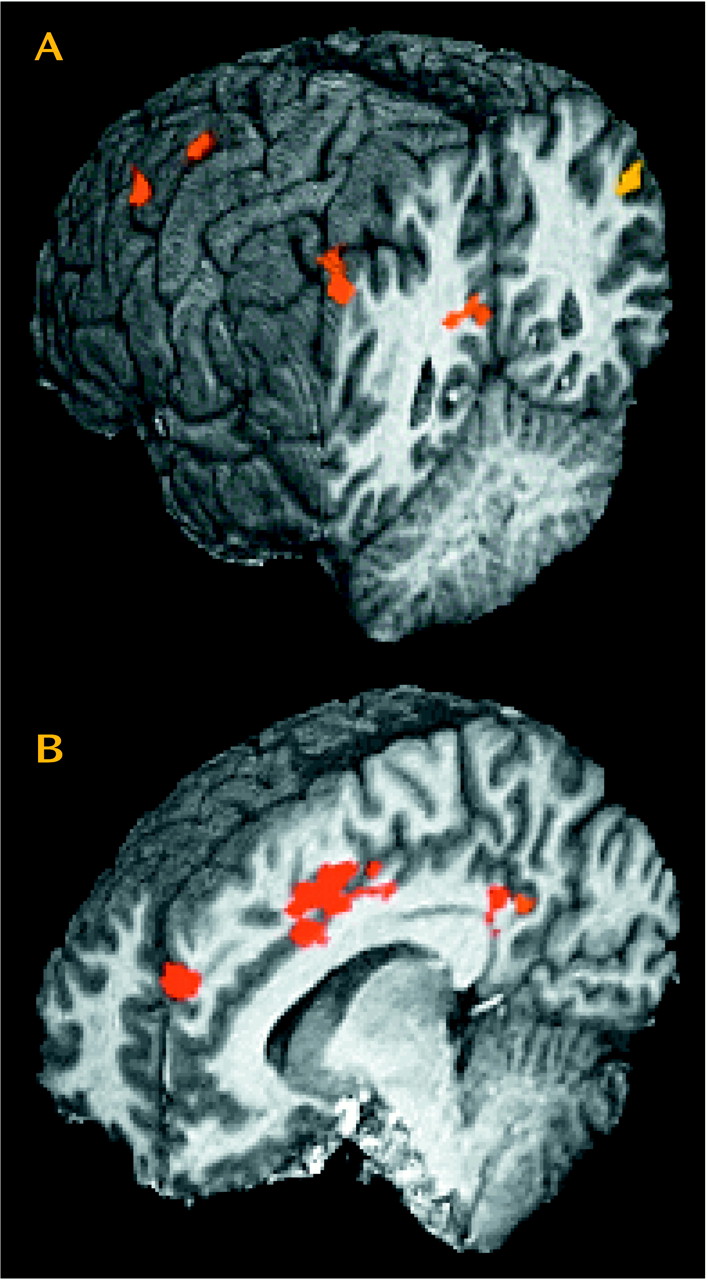
Most (13 of 19) of these regions of interest showed significantly greater activation in cocaine users than in comparison subjects when comparing activation induced by the cocaine film (
Table 3 and
Figure 2). These same 13 regions of interest also demonstrated significantly greater activation for cocaine users viewing the cocaine film when compared to cocaine users viewing the nature film. By not being present in comparison subjects viewing the same cocaine film content and not being present in users viewing the nature film content, these results speak to the specificity of these 13 regions for cocaine craving.
Conversely, only a minority of these regions of interest (four of 19) showed significantly different activation when cocaine users viewing the cocaine film were tested against cocaine users viewing the sex film, and one of these four, located in the left inferior frontal gyrus, was not significant in either of the two previous comparisons (
Table 3). The remaining three regions of interest, which were significant in all three comparisons, were centered on the anterior cingulate gyrus, the right inferior parietal lobule, and the left caudate/lateral dorsal nucleus. Finally, just four of the 19 regions of interest showed significant differences between cocaine users viewing the cocaine film and comparison subjects viewing the sex film. All four regions of interest showed greater activation in the cocaine users but just one, located in the right inferior parietal lobule, had also been significant in the previous comparisons. The remaining three regions of interest were located in the right superior frontal gyrus, left inferior parietal lobule, and the anterior nucleus of the thalamus. In interpreting these results, it should be noted that these between-group and between-film comparisons may be biased toward the activation map of cocaine users viewing the cocaine film, since it was this condition that functionally defined the regions of interest.
Functional Activation Analyses: Sex and Nature Films
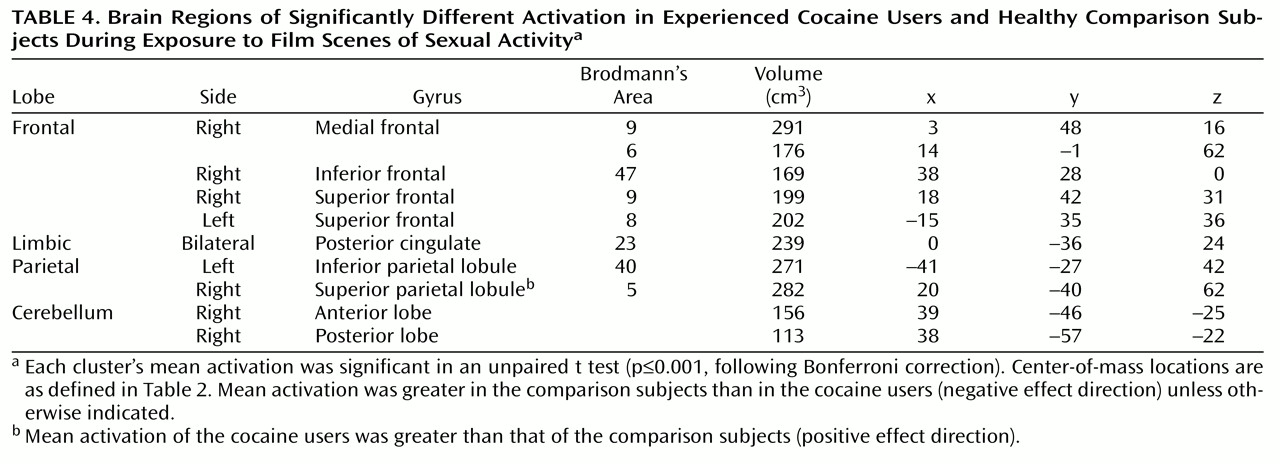
Similar regions were activated in cocaine users and comparison subjects when they viewed the sex film. These included extensive frontal (medial, superior, and inferior frontal gyri), anterior and posterior cingulate, bilateral insula, caudate, thalamic, occipital, and cerebellar regions. More clusters (36 versus 25), encompassing a greater total volume (8,942 μl versus 5,280 μl), and having a greater average activation (0.24% versus 0.20%) were observed in the comparison subjects than in the cocaine users. The activated clusters of both groups were combined and t tests were performed that compared the two groups on the mean activation values of each of the combined clusters. Of the 52 clusters in this combined map, 29 showed significant differences, and of these, 23 showed significantly greater activation in the comparison subjects than in the cocaine users. A Bonferroni correction (p≤0.001), warranted given the high number of separate statistical tests, reduced the number of significant differences to 10. Half of these clusters were located in the frontal lobes, and half were in the cerebellum, posterior cingulate, and parietal lobes (
Table 4). Of these 10 clusters, nine showed greater activation in the comparison subjects than in the cocaine users. Allowing for the fact that the comparison subjects contributed more clusters to the combined map and accounting for the high number of statistical tests, these analyses show a greater responsiveness on the part of the comparison subjects to the sex film than was shown by the cocaine users. This stands in contrast to the results from the cocaine film analyses.
Few regions were significantly activated in either the cocaine users (four clusters) or the comparison subjects (two clusters) when viewing the nature film. Two clusters, located in the right posterior cingulate and right fusiform gyrus, were significantly greater in comparison subjects than in the cocaine users, while two others, located on the right superior frontal gyrus (Brodmann’s area 9) and the left postcentral gyrus (Brodmann’s area 3), showed the opposite pattern.
Discussion
Based upon self-reports of the experienced cocaine users, viewing a film that depicted two men smoking crack cocaine was sufficient to induce cocaine craving. Functional MRI analyses revealed a distribution of brain regions that showed a significant increase in activation in the cocaine users as the cocaine cues were being viewed. These regions were in the prefrontal (medial and dorsolateral), limbic (anterior and posterior cingulate), and parietal (bilateral inferior parietal lobule) lobes. The right insula and left temporal pole were also activated. A number of control comparisons were performed to determine the specificity of these activated regions for cocaine craving. First, the majority of responsive areas were activated to a significantly greater extent in cocaine users than in comparison subjects during the cocaine film, which suggests that the response shown by the cocaine users was not reflecting an inherently evocative characteristic of the film but instead was contingent upon the subject having a history of cocaine use. Second, these same brain areas were more activated in cocaine users watching the cocaine film than in cocaine users watching the nature film, which demonstrates that the responsive areas in the cocaine users were not trait-like responses indiscriminately induced by any film but instead were specific to the cocaine film’s content. Combined, this population and content specificity help to isolate the critical neuroanatomical substrates of cue-induced cocaine craving.
Neuroanatomical Localization of Cue-Induced Cocaine Craving
Cocaine craving was associated with a widespread pattern of cortical activation that was largely consistent with previous neuroimaging reports of cue- and drug-induced craving. Both dorsolateral prefrontal
(12–
15) and anterior cingulate activation
(13,
15–
17,
26) have been consistently seen. Temporal pole activation is consistent with a recent report of increased cerebral blood flow in this region when cocaine users watched a cocaine film relative to a nature film
(17), and parietal lobe activation related to cocaine craving has also been reported
(14,
26).
We interpret the distributed activation in cocaine users as witness to the contribution of multiple, distinct psychological processes, both cognitive and emotional, to creating the craving state. For example, given that many neuroimaging studies have observed frontal and parietal co-activation during working memory
(27–
30) and attentionally demanding tasks
(31,
32), the activation seen in these structures in the present study might suggest the participation of a frontoparietal working memory circuit in craving or a heightened attention to the cocaine film by users. The implication of this finding is that the engagement of the drug user’s attention and his or her subsequent drug-related ruminations, mediated within a working memory system, may be critical for the initiation and maintenance of the craving state. Such a finding may speak to the appropriateness of therapeutic approaches that seek to ameliorate drug craving through attention redirection and subvocal rehearsal techniques.
Activation of the anterior cingulate has been frequently observed during cocaine craving and is thought to play an integral role in both cognitive and affective processes
(33,
34). A review contrasting cingulate activations for cognitive/motor tasks and emotional/symptom provocation localized the former to an anterior region that includes the cingulate activation observed in the present study
(35). Posner and Rothbart
(36) have proposed that the cingulate plays a critical role in the executive control or regulation of emotional states. Medial frontal regions have also been proposed to subserve emotional processes
(37,
38), which may explain the activation seen in these regions in cocaine users during the cocaine film, a supposition supported by the medial frontal activation in both groups of subjects during the sex film. In a review of studies that addressed film- and recall-generated emotions as well as anticipatory anxiety, Reiman
(39) concluded that the anterior cingulate and medial prefrontal region (Brodmann’s area 9) participate in “the conscious experience of, attentional response to, or behavioral response to the anxiety provoking situation.” It is plausible to conclude, therefore, that the anterior cingulate and medial prefrontal activations of the present study reflect the participation of these emotional and attentional mechanisms in cocaine craving.
The posterior cingulate region activated during cocaine craving may reflect the participation of a “normal” endogenous drive state or craving response, since this region was also shown to be the most active in a correlation with hypertonic saline-induced thirst and subsequent satiation in normal control subjects
(40). Alternatively, Vogt and colleagues
(34) assigned an evaluative role in assessing the environment and a memory role to the posterior cingulate. Retrosplenial activation has been previously observed during cue-induced craving
(14) and during presentation of threat-related words
(41) and in both cases has been interpreted to reflect episodic memory processes, consistent with the suggestion that this area may be specifically involved in the recall of emotional memories
(42).
The post hoc assignation of psychological function to regional activation is somewhat speculative and awaits corroboration from experiments that address the specific role of a particular psychological process in the functional anatomy of cocaine craving. Nonetheless, the pattern of activation that has been observed to be specific to the cocaine users and to the content of the cocaine film suggests that the craving response is plausibly manifested through the same circuitry that has been observed in other experiments in normal drug-naive subjects. It is to be expected that the cognitive and affective processes that have been previously described in nondrug experimental paradigms are the same processes subserved by the same brain regions from which the craving response emerges.
Comparison With Response to Sexual Content
For the cocaine users, a minority of the observed craving sites significantly differed in activation in response to the cocaine and sexual stimuli. Furthermore, just four of the observed craving sites differed in activation when contrasted with the comparison subjects’ responses to the sexual stimuli. Together, these findings suggest a large overlap in the brain circuitry that underlies responsivity to cocaine-related stimuli and to other nondrug, evocative stimuli. This overlap includes all the medial frontal regions, most of the remaining dorsal frontal regions, as well as most of the cingulate regions. Although just one region of interest, located on the right inferior parietal lobule, did appear to be most specific for cocaine craving by being significant in all the planned comparisons, we would caution against concluding that it constitutes the essence of cocaine craving. Rather, we interpret these results to mean that of those regions of interest identified as the neuroanatomical substrates for cue-induced cocaine craving, most were also responsive to other evocative stimuli and were so for both drug users and drug-naive comparison subjects. This does not necessarily diminish the role of these less-specific regions in cocaine craving but instead suggests that the cocaine craving response is not produced by a circuitry unique to the cocaine user. Rather, it is what activates this circuitry that is unique to the cocaine user. A fuller understanding of these uniquely large responses may shed some light on how prolonged cocaine usage affects normal brain systems for desire and leads to the devastating disorder known as cocaine dependency.
It has been suggested that cocaine’s potency may stem from its ability to directly activate the mesocorticolimbic dopamine system
(43). It has been demonstrated that chronic cocaine self-administration in rats produces increased brain stimulation reward thresholds during subsequent withdrawal
(44). In a similar manner, chronic drug use may reduce the reinforcing efficacy of natural stimuli; anecdotally, experienced drug users typically report a preference for cocaine over sex. In the present study, most regions identified as cocaine craving sites were similarly activated by sexual stimuli (more precisely, were not significantly different in activation between the two films). Furthermore, cocaine users showed diminished activation relative to comparison subjects in their respective responses to the sex film. These findings may have important clinical consequences. If cocaine craving is subserved by the same brain regions that are activated by “naturally” rewarding/evocative stimuli, then this could result in a rewriting of “normal” emotionally driven preferences
(45). If cocaine not only acts upon but has co-opted the brain’s reward circuits, thus leading to a rewriting of normal emotionally driven preferences, then this may have serious consequences for the decision making of cocaine users. The attenuated response to normal rewards may be exacerbated when in a craving state, thus further feeding the specific desire for cocaine.
It should be noted that the smaller neuroanatomical response of the cocaine users to the sexual stimuli relative to the comparison subjects was not reflected in the self-reports that followed the sex film in which, in general, no differences were observed between the groups. One possibility is that the users, who had already been quite frank concerning their drug use, were more honest in their appraisal of the sex film (the suggestion is that the comparison subjects may have underreported the extent to which they liked the sex film). Given that the self-report questions were constructed with the assessment of the cocaine film to the fore, a more sophisticated probe of each film’s effects, for example, one that pitted the reinforcement value of cocaine against that of sex (as in a forced choice between which film, sex or cocaine, to continue viewing), may have yielded behavioral indices that better mirrored the brain activations. An alternative interpretation may accept the apparent dissociation as indicative of a “normal,” conscious (i. e., verbalizable) appreciation of the sexual material but an impaired neurological capacity to enjoy it, with the implication that this is a “trait or state” consequence of years of drug use.
Conclusions
Cue-induced cocaine craving is often cited as a major determinant in drug relapse. We have reported a distributed pattern of cortical activation, primarily prefrontal and limbic, that presumably reflects the cognitive and emotional processes that participate in the cue-induced craving state. Further research should be able to disentangle the relative influences of these separate processes. One speculation, out of a number of possibilities, is that the cingulate and medial prefrontal activations may provide the emotional tone of the craving response while the dorsal prefrontal and parietal areas may be involved in an increased attentional processing of, or working memory ruminations upon, the cocaine stimuli. Identifying the relative importance and level of interdependence of the processes that constitute craving should help optimize therapeutic interventions for blocking craving and mitigating consequent drug-seeking behavior. The majority of regions identified as craving sites exhibited similar responses to the sexually explicit material, thus implicating common circuits in drug and nondrug reactivity. Taken together, these results are consistent with the hypothesis that cocaine acts upon normal reward/emotional circuitry and that cocaine craving rests upon the user’s memory for cocaine’s reinforcing effects. On an optimistic note, this suggests that what is already known of normal learning, memory, and emotions may be usefully applied to an understanding of cue-induced craving and may inform appropriate pharmacological and behavioral/cognitive interventions.