Cognitive impairments are central features of schizophrenia and are related to functional status and other aspects of the illness
(1). These deficits have been recognized for years but their status as important features of the disorder has recently received greater attention. Cognitive deficits in schizophrenia are not completely caused by the symptoms of the illness or aspects of its treatment, and they are stable over time across fluctuations in other aspects of clinical state
(2). Some studies have suggested that these deficits may be partly responsive to treatment with novel antipsychotic medications
(3), although they appear to be quite refractory to treatment with conventional agents
(4). First-episode patients appear to have deficits in their functioning that are similar to those seen in patients with chronic illness
(5,
6), although patients with poor outcome show some signs of deterioration in cognitive functioning in later life
(7).
Although cognitive impairments are common in schizophrenia, they are not specific to this illness. Subjects with affective disorders also perform worse than normal comparison subjects or normative standards on many measures of cognitive functioning
(8–
10), as do subjects with other neuropsychiatric conditions such as head trauma, cerebrovascular accidents, seizure disorders, and dementia
(11). Comparisons of the profile and magnitude of the cognitive deficits of patients with schizophrenia with those of patients with other neuropsychiatric disorders can provide information about potential etiological factors and neurobiological substrates of the deficits. For example, neuropsychological and neuropathological studies that discriminate between subjects with Alzheimer’s disease and those with schizophrenia have suggested that the cognitive impairments in these illnesses have different biological substrates
(12,
13), as have comparative studies contrasting subjects with schizophrenia and subjects with temporal lobe epilepsy
(14).
When patients with affective disorders were compared with patients with schizophrenia, a somewhat contradictory pattern of results emerged. Some reports suggested that subjects with affective disorders perform better overall on neuropsychological tests than subjects with schizophrenia
(15). Others found limited differences in performance between the two diagnostic groups
(16–
24), especially in subjects with chronic illness or poor outcome
(20). Finally, two studies that distinguished between psychotic and nonpsychotic affective disorders reported significant differences between patients with schizophrenia and those with nonpsychotic affective disorders but not between patients with schizophrenia and those with psychotic affective disorders
(25,
26).
This report compares the performance of subjects with schizophrenia, psychotic major depressive disorder, and psychotic bipolar disorder on a comprehensive neuropsychological assessment battery. Several features of this study are important. The study group was composed exclusively of first-admission patients who had psychotic symptoms at the time of admission. Consensus diagnoses were based on structured interviews and other information gathered over the 2-year follow-up in order to make a reliable distinction between major depressive disorder and bipolar disorder. Neuropsychological assessment was conducted 2 years after the index admission. The individual tests in the battery were selected on the basis of both relevance to schizophrenia and the availability of comprehensive norms. Finally, statistical analyses compared performance both globally and specifically on individual tests and identified profiles of performance for subjects with schizophrenia and for subjects with psychotic affective disorders.
Results
Background and Illness Characteristics
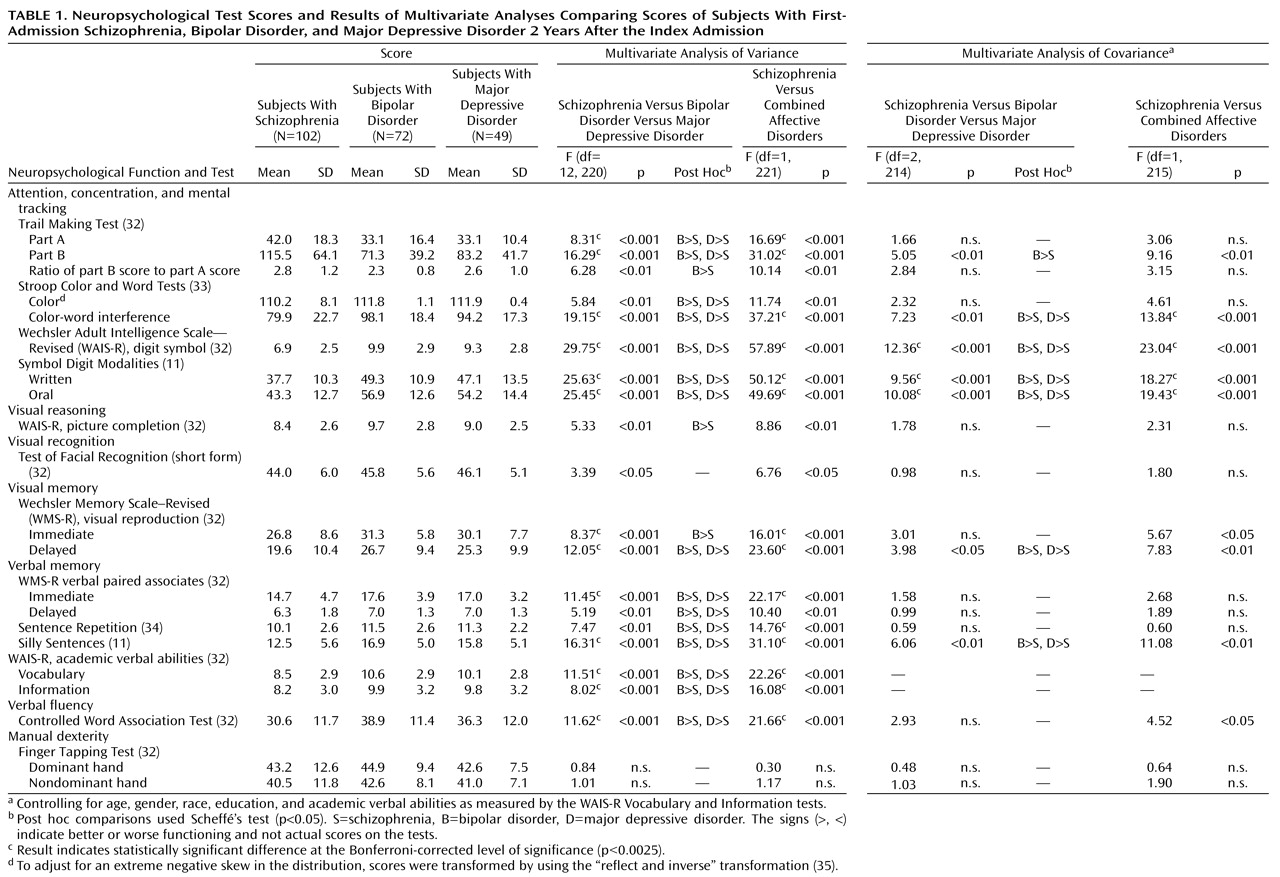
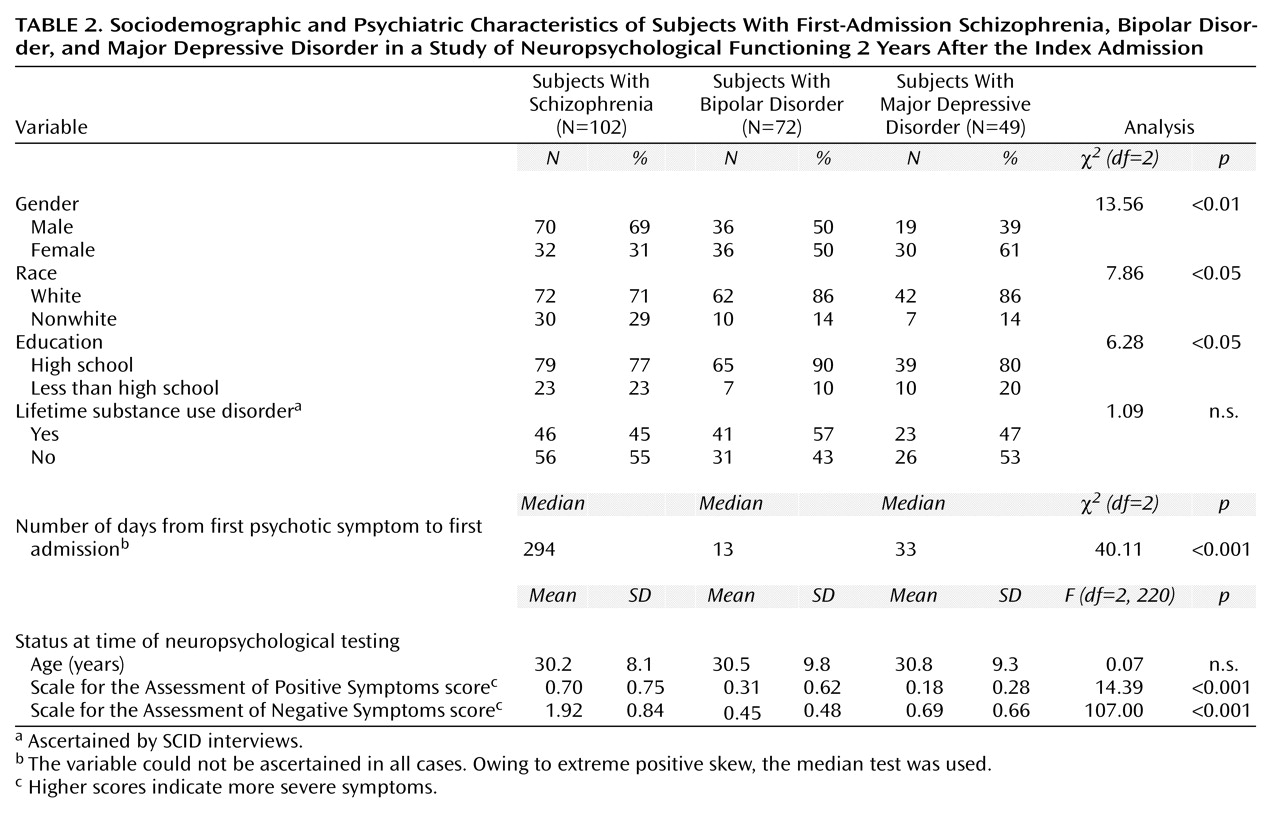
The sociodemographic and psychiatric characteristics of the subjects are presented in
Table 2. Compared to subjects in the two psychotic affective disorder groups, subjects in the schizophrenia group were more likely to be male and nonwhite. They also had higher levels of both positive and negative symptoms, were more likely to be taking medications at the time of testing, and had a longer time lag between their first psychotic symptom and first admission. Subjects with bipolar disorder were more likely than those with either schizophrenia or major depressive disorder to be high school graduates. There were, however, no diagnostic differences in age at the time of testing and in substance use comorbidity.
Diagnostic Comparisons
Means and standard deviations for the three diagnostic groups’ neuropsychological test scores are presented in
Table 1. Overall, there was a statistically significant difference among the groups in their performance on the neuropsychological tests (Pillai’s test=0.33, F=1.87, df=42, 402, p<0.01). For 13 of the 21 comparisons, the differences reached the Bonferroni corrected level of significance (p<0.0025). The results of the post hoc comparisons revealed that the schizophrenia group did significantly worse than each of the affective disorder groups. However, there were no significant differences between the bipolar disorder group and the major depressive disorder group. As a result, the analysis was repeated after combining the two affective disorder groups. This second MANOVA also showed a statistically significant overall difference between the schizophrenia group and the combined affective disorders group (Pillai’s test=0.29, F=3.83, df=21, 201, p<0.001). In addition, the subjects with schizophrenia performed significantly worse on the same individual tests in the two-group comparison as in the three-group comparison.
Diagnostic Comparisons Adjusting for Covariates
To select covariates, we first examined the relationship of demographic characteristics to performance on the neuropsychological tests. These analyses showed that the following characteristics were associated with neuropsychological test performance: age at the time of testing (Pillai’s test=0.28, F=3.70, df=21, 201, p<0.001), gender (Pillai’s test=0.39, F=6.15, df=21, 201, p<0.001), race (white versus nonwhite) (Pillai’s test=0.22, F=2.76, df=21, 201, p<0.001), and education (Pillai’s test=0.24, F=2.95, df=21, 201, p<0.001). On the other hand, medication status (receiving psychoactive medications at the time of testing) (Pillai’s test=0.14, F=1.50, df=21, 201, n.s.) and whether the physical conditions of testing were favorable (Pillai’s test=0.13, F=1.44, df=21, 201, n.s.) were not significantly associated with neuropsychological performance. Because too few subjects were abusing substances at the time of testing, we could not reliably assess the impact of this variable. However, lifetime substance use disorder was not significantly associated with performance (Pillai’s test=0.14, F=1.57, df=21, 201, n.s.). In addition, there was no association between the interval from the first psychotic symptom to the first hospitalization (log transformed owing to substantial positive skew) and neuropsychological performance (Wilks’s test=0.88, F=1.31, df=21, 193, n.s.).
On the basis of these analyses, age, gender, race, and education were selected as covariates in the MANCOVA. Scores on the Information and Vocabulary tests of the WAIS-R were also included as covariates to control for the effect of general intellectual functioning. Two MANCOVAs were conducted, one comparing the three diagnostic groups and the second comparing the schizophrenia group with the combined affective disorder group (
Table 1). The results of the first MANCOVA did not indicate a statistically significant overall difference between the three diagnostic groups (Pillai’s test=0.19, F=1.10, df=38, 394, n.s.). The overall test result for comparison of the schizophrenia and the combined affective disorder groups in the second MANCOVA, however, was statistically significant (Pillai’s test=0.15, F=1.84, df=19, 197, p<0.05). Inspection of the results for the individual comparisons in both MANCOVAs revealed that the schizophrenia group had significantly worse performance than both affective disorder groups, considered separately or in combination on measures of attention, mental tracking, immediate nonsemantic verbal memory (Silly Sentences), and delayed visual memory. As before, the two groups with affective disorders did not perform significantly differently on any tests.
Only seven (10%) of the 72 subjects with bipolar disorder and three (6%) of the 49 subjects with major depressive disorder, compared with 36 (35%) of the 102 subjects with schizophrenia, had psychotic symptoms at the time of testing (χ
2=24.9, df=2, p<0.001). We thus repeated the final MANCOVA for the subjects with no current psychotic symptoms at 24-month follow-up (N=177). Although the overall test comparing the schizophrenia group and the combined affective disorder group was not significant (Pillai’s test=0.17, F=1.61, df=19, 151, n.s.), the pattern of significant findings for individual comparisons (results not shown) was the same as that reported for the full sample, presented in
Table 1.
Discriminant Function Analysis
Eleven neuropsychological tests were included in the discriminant analysis, including the WAIS-R Vocabulary and Information tests, along with the nine tests that showed a significant difference (p<0.05) between the schizophrenia group and the combined affective disorders group in the MANCOVA (
Table 1, last two columns). Using a stepwise method, the final model retained three measures: Digit Symbol, Symbol Digit Modalities—oral, and Silly Sentences. One discriminant function that was based on these three variables accounted for more than 95% of the between-group variance (Wilks’s lambda=0.75, p<0.001). The model correctly classified 73% (N=74) of the subjects with schizophrenia and 75% (N=91) of the subjects with psychotic affective disorders.
Discussion
The major finding of this study was that at 2-year follow-up, first-admission subjects with schizophrenia performed worse on a number of neuropsychological tests compared with subjects with psychotic affective disorders, even after the analysis controlled for demographic variables and general intellectual functioning. Furthermore, the differences appeared to follow a particular pattern, in which subjects with schizophrenia performed consistently worse on tests of attention, concentration and mental tracking, visual memory, verbal fluency, and nonsemantic verbal short-term memory.
Deficits in attention and concentration have been implicated as central deficits in schizophrenia from early times
(36,
37). More recently, Pearlson et al.
(38) proposed involvement of the heteromodal association cortex as the neurological substrate for a range of neuropsychological deficits in schizophrenia. The heteromodal association cortex is a diffuse functional unit that includes interconnected regions in the prefrontal, temporal, and parietal cortex and subserves a host of higher cortical functions, including focused attention, working memory, and verbal fluency. This hypothesis is further supported by the results of studies linking neuropsychological deficits with negative symptoms
(39,
40). These symptoms are characterized by deficits in motivation and socialization, functions also attributed to the heteromodal association cortex system. Work by Hoff et al.
(41,
42) has further suggested that these neuropsychological deficits—along with negative symptoms—are apparent early in the course of schizophrenia and persist over the long term. Thus, these deficits might represent trait markers for the illness.
The specificity of these deficits to schizophrenia was supported in a magnetic resonance imaging study
(43) comparing subjects with schizophrenia, subjects with bipolar disorder, and matched normal comparison subjects. That study showed reduction in gray matter volume in heteromodal association cortex regions in schizophrenia but not in bipolar disorder. Our findings further support the hypothesis of dysfunction in the heteromodal association cortex in schizophrenia by revealing specific deficits in neuropsychological functions served by this system.
Three neuropsychological tests (Digit Symbol, Symbol Digit Modalities—oral, and Silly Sentences) explained a large proportion of the variation in neuropsychological functioning between the schizophrenia group and the psychotic affective disorders group. The Digit Symbol is a test of motor persistence, sustained attention, response speed, and visuomotor coordination
(11). Symbol Digit Modalities—oral is primarily a test of complex scanning and visual tracking and is highly correlated with the Digit Symbol
(11). Silly Sentences, a test of immediate nonsemantic verbal memory, assesses the contribution of meaning to retention
(11).
The Digit Symbol test has been administered in three previous comparative studies
(18,
25,
26). In the study by Morice
(18), a comparison of the mean Digit Symbol scores of chronically ill treatment-seeking subjects with schizophrenia (mean score=5.7) and with bipolar disorder (mean score=6.5) was not statistically significant. Moreover, these chronically ill subjects performed worse than the subjects in the present study. Jeste et al.
(25) compared schizophrenia subjects to psychotic and nonpsychotic depression subjects. All were over 45 years old. The subjects with schizophrenia and those with psychotic affective disorders had mean scores of 8.1 and 8.4, respectively, whereas the performance of the nonpsychotic affective disorder group was consistent with normative standards. Finally, Albus et al.
(26) compared first-episode schizophrenia subjects to psychotic and nonpsychotic affective disorder subjects. The researchers combined the Digit Symbol test, the Stroop Color and Word Tests, and the Trail Making Tests to form a “visual motor processing and attention” dimension. Although test score means were not provided, the subjects with schizophrenia scored 2.2 standard deviations lower on this dimension than the normal comparison group and 1.4 standard deviation lower than the nonpsychotic affective group, but only 0.3 standard deviations lower than the psychotic affective group.
The performance of subjects with bipolar disorder in the sample described here was indistinguishable from that of subjects with major depressive disorder. The neuropsychological performance of these groups has rarely been directly compared. However, given the differences in the course and clinical outcome of these disorders
(27), one might expect differences in the neuropsychological functioning as well. Our finding of no difference in the neuropsychological performance of these two groups needs to be confirmed in future studies.
The results of this study should be interpreted in light of its limitations. First, we did not have a normal comparison group. However, almost all of the tests in our battery were well known and had widely available standard norms. Comparison with these norms in many cases confirmed poorer functioning, especially in the group with schizophrenia.
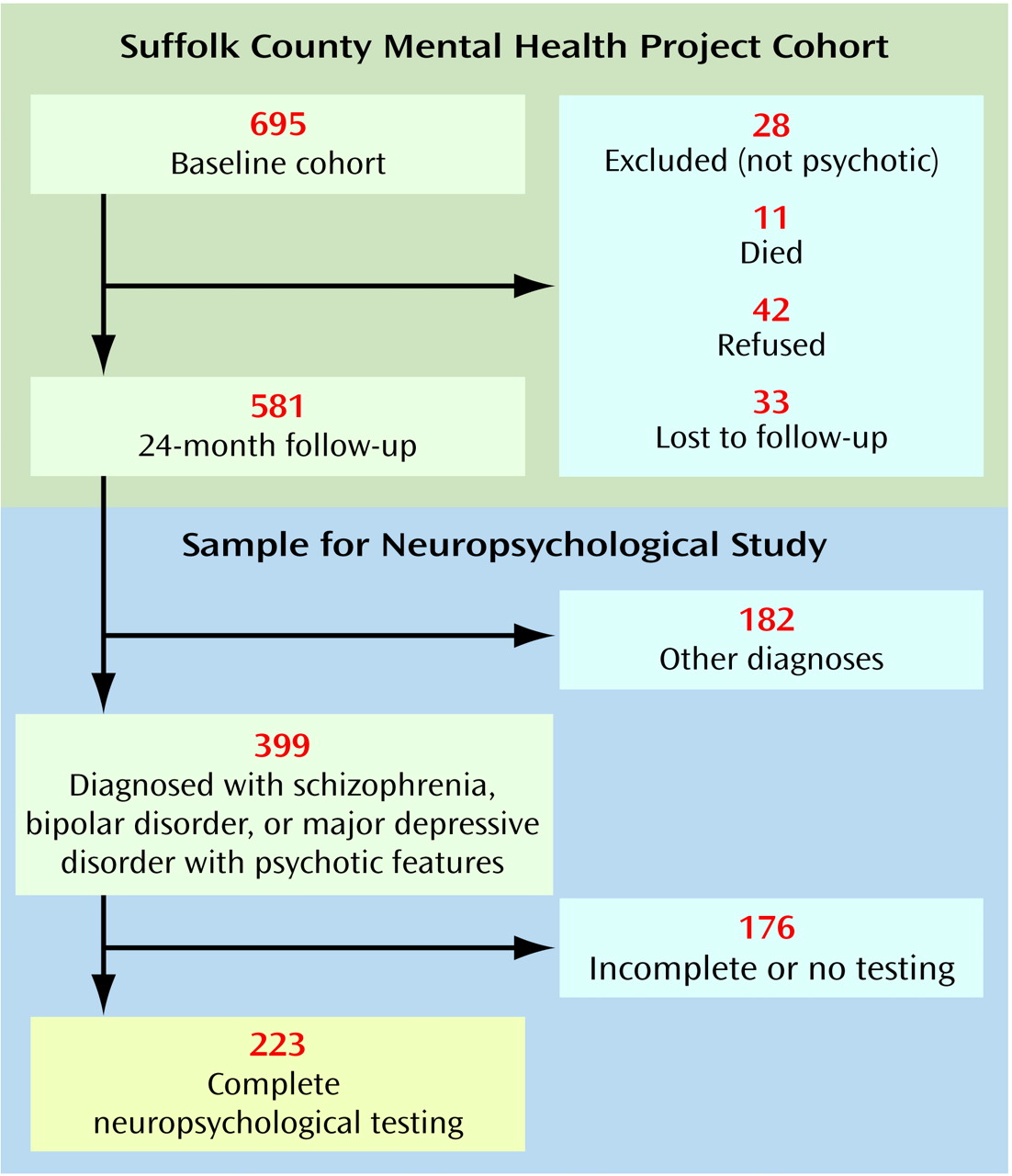
Second, as presented in
Figure 1, 42 subjects refused the 24-month follow-up interview and another 33 were lost to follow-up. However, except for age, these 75 were not different from the 581 who were successfully followed up in demographic characteristics, baseline diagnosis, or other psychiatric variables. Subjects who refused or were lost were slightly older than those who were contacted (mean age=33.3 years, SD=10.6, and 29.4 years, SD=9.5, respectively) (t=3.29, df=654, p<0.01).
Third, 176 respondents did not have a complete neuropsychological assessment because they were interviewed by phone or mail, were too disturbed to be tested during the interview, or failed to complete one or more components of the assessment. There were few differences, however, between the 223 respondents who completed the battery and the 176 who did not. Subjects with schizophrenia who did not complete the neuropsychological evaluation had more severe positive symptoms compared with the subjects with schizophrenia who completed the battery (SAPS mean score=1.05, SD=0.96, and 0.70, SD=0.75, respectively) (t=2.35, df=143, p<0.05). Furthermore, subjects with bipolar disorder who did not complete the neuropsychological evaluation had more severe negative symptoms compared to subjects with bipolar disorder who completed the battery (SANS mean score=0.79, SD=0.81, and 0.45, SD=0.48, respectively) (t=2.60, df=99, p<0.05). A similar result for negative symptoms was obtained in comparing subjects with major depressive disorder who completed the neuropsychological battery with those who did not (SANS mean score=1.42, SD=1.16, and 0.69, SD=0.66, respectively) (t=2.84, df=58, p<0.01). However, neither positive symptoms in subjects with schizophrenia nor negative symptoms in subjects with bipolar disorder or major depressive disorder were associated with neuropsychological performance in these diagnostic groups. Therefore, it is unlikely that exclusion of these 176 subjects biased the results of comparisons between diagnostic groups.
Finally, some authors
(37,
44) have cautioned that differences in psychometric properties of tests make interpreting profiles of performance across groups less reliable than interpreting global differences. Caution is therefore necessary in interpreting the different patterns of deficits found in this study. However, it is reassuring that we obtained consistent results across different tests of the same domains of neuropsychological functioning (e.g., attention, concentration, and mental tracking). Another consideration when interpreting the different patterns of test performance across diagnostic groups is the possibility that certain abilities or tests for these abilities (in this case, tests of attention, concentration, and mental tracking) are more sensitive to global nonspecific deficits than are other abilities
(37).