The neural circuits that mediate cue responsivity and craving may be accessible with functional neuroimaging techniques. For example, cocaine-related cues and cocaine craving have been shown to induce changes in regional cerebral activation in diverse brain regions. Brain areas that are reported to show activations consistently across paradigms include the dorsolateral prefrontal cortex
(6,
7), anterior cingulate cortex
(7,
8), amygdala
(6,
8), and orbitofrontal cortex
(9,
10). The orbitofrontal cortex is also activated in response to heroin-related cues
(11). Animal models of drug dependence have implicated many of these brain regions in the positive reinforcing effects of drugs, the negative reinforcing effects of withdrawal, and the ability of environmental stimuli to become conditioned stimuli for both of these effects
(4).
We predicted that in opiate-dependent individuals, opiate-related autobiographical cues would activate brain regions associated with responses to opiate or cocaine cues and craving, i.e., the limbic system, in particular the anterior cingulate cortex, the amygdala, the dorsolateral prefrontal cortex, and the orbitofrontal cortex.
Method
Subjects
Twelve subjects (11 men, one woman; mean age=34.4 years, range=27–45 years) with a history of opiate dependence (mean duration=7 years, range=4 months–20 years) were recruited from drug treatment agencies. All were opiate free, as confirmed by urine testing, at the time of the study. The duration of abstinence from opiates before the study was between 10 days and 3 years (precise data were unavailable for two subjects, the mean for the other 10 subjects was 8.25 months). All subjects were right handed. Although seven subjects had a history of abuse of other drugs, none of those subjects was using other illegal drugs at the time of the study. Their status was confirmed by urine testing. Two subjects were taking selective serotonin reuptake inhibitor antidepressants, and one was taking anticonvulsants. No other psychoactive medication was permitted, but one subject was taking the anticholinergic drug oxybutynin for detrusor instability. This study was approved by the local ethics committees and the Administration of Radioactive Substances Advisory Committee. After a complete description of the study, written informed consent was obtained from the subjects.
Protocol
Subjects recorded two audiotaped scripts before the positron emission tomography (PET) scanning session. Each script lasted 2 minutes and recounted a single specific episode from the subject’s past. One of the two scripts described an episode when the subject experienced a strong craving for opiates; the other described a neutral episode. Subjects were specifically told to focus on describing the feelings, both emotional and visceral, of the opiate craving they experienced during the episode they were recounting. Subjects then underwent a 12-run PET scan of regional cerebral blood flow (rCBF) with the tracer [
15O]H
2O administered with a slow bolus technique. There was a 10-minute gap between runs; the total scan lasted 2 hours. A brain-dedicated ECAT 953b PET camera (CTI PET Systems, Knoxville, Tenn.) operating in a high-sensitivity three-dimensional mode was used. During each of the 12 runs, the subject listened to one of the two audiotaped scripts. Each drug-related and neutral script was presented six times, in random order. Image acquisition began 90 seconds after the beginning of the script to allow craving to be induced in response to the stimulus. Images were acquired in a single 90-second frame. Radioactivity counts acquired during this time frame predominantly reflect changes in rCBF during the rising phase of radioactivity in the head, which typically lasts 30–45 seconds. As described previously
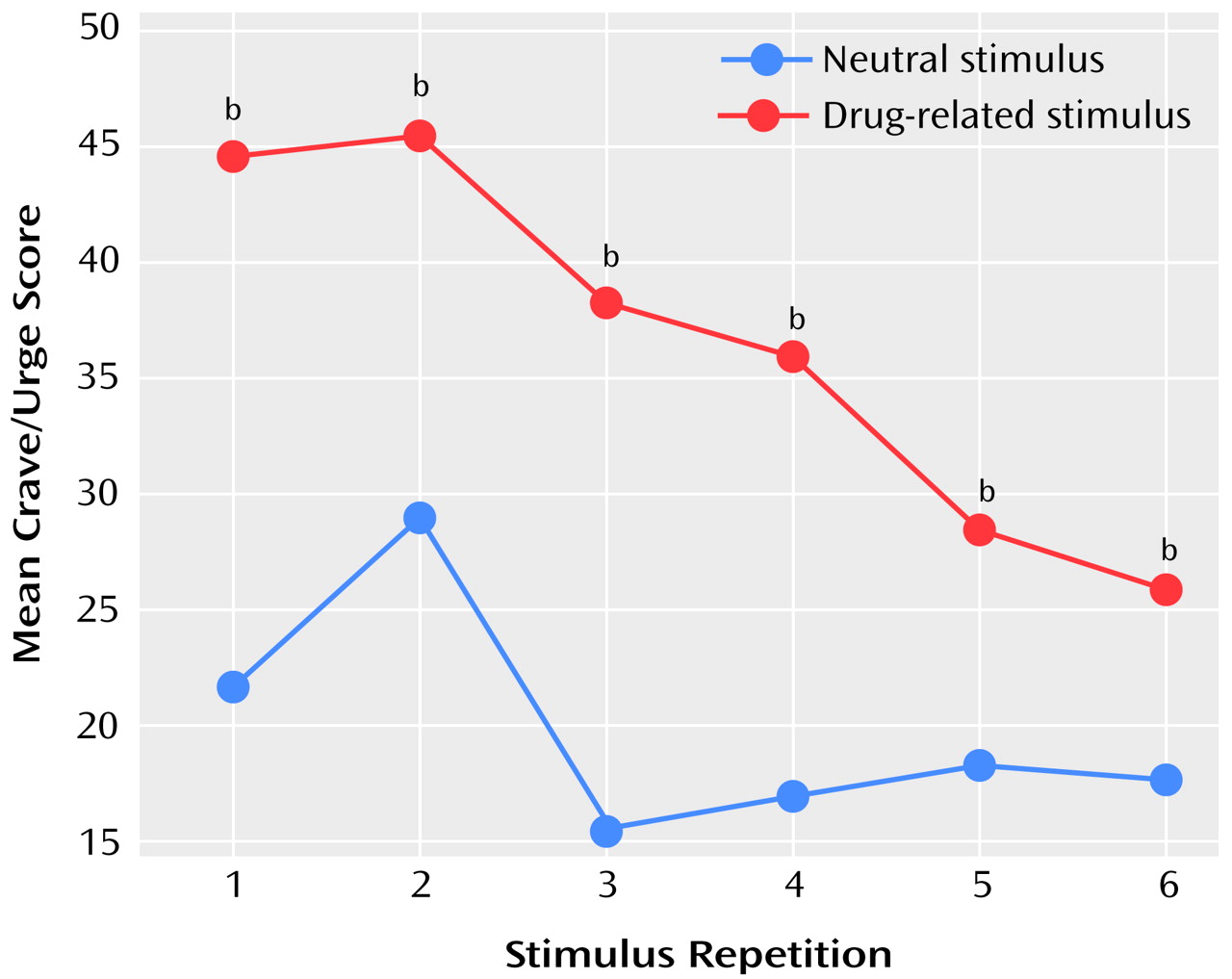
(12), after each run the subjects completed six visual analogue scales measuring craving for heroin, urge to use heroin, happiness, sadness, anxiety, and vividness of the script.
Image Analysis
The PET images of rCBF were analyzed with statistical parametric mapping software SPM99 (Wellcome Department of Cognitive Neurology, University College London). The 12 images for each subject were realigned to a mean image for that subject to partially correct for motion of the subject between scans. The realigned images were normalized to a standardized template [15O]H2O PET image. The resultant realigned normalized images were then smoothed with a Gaussian function at 12 mm full width at half maximum.
Statistical analysis of the images was done by using cognitive subtraction and a correlational analysis. For the cognitive subtraction, a condition comparison was carried out, with the audiotaped scripts (drug-related or neutral) determining the conditions. For the correlational analysis, the craving and urge-to-use visual analogue scale scores were averaged to produce a more normally distributed composite crave/urge scale. This composite crave/urge scale was then used as a covariate of interest to examine areas of rCBF that covaried with this scale. This second analysis included only those subjects who reported craving in response to the cue-exposure stimuli. Four subjects were excluded from this analysis since their crave/urge scale scores did not vary from zero throughout the scanning session. To include those subjects who did not report any craving, i.e., whose visual analogue scale scores did not vary, would contravene the basic assumptions of normal distribution that underlie the statistics in statistical parametric mapping, which uses the general linear model.
In the statistical parametric mapping analyses, time from the start of the first scan was entered as a covariate of no interest to take account of any rCBF changes that were related to nonspecific factors. All covariates were centered around subject means and entered with a subject-specific fit. Global cerebral blood flow effects were removed by an analysis of covariance model that allowed for subject-specific fit. The threshold for statistical significance for all analyses was set at p<0.05 after correction for multiple comparisons for either peak change in rCBF or the size of the activated cluster.
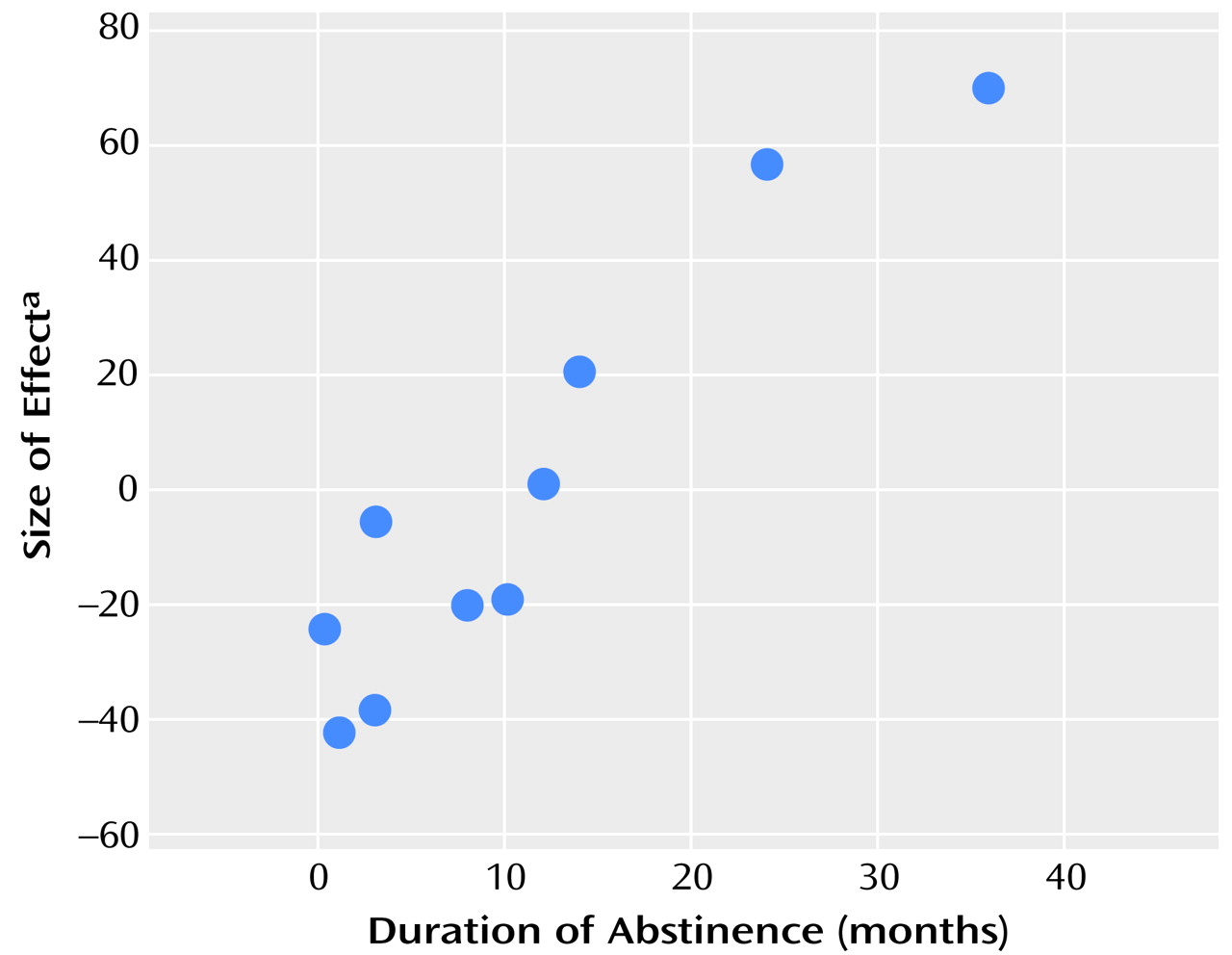
In a final analysis, the contrast, or difference, images for each subject comparing the effects of the two conditions were entered into a second-level analysis to examine the effects of duration of abstinence. The two subjects for whom duration of abstinence was not precisely known were excluded.
Discussion
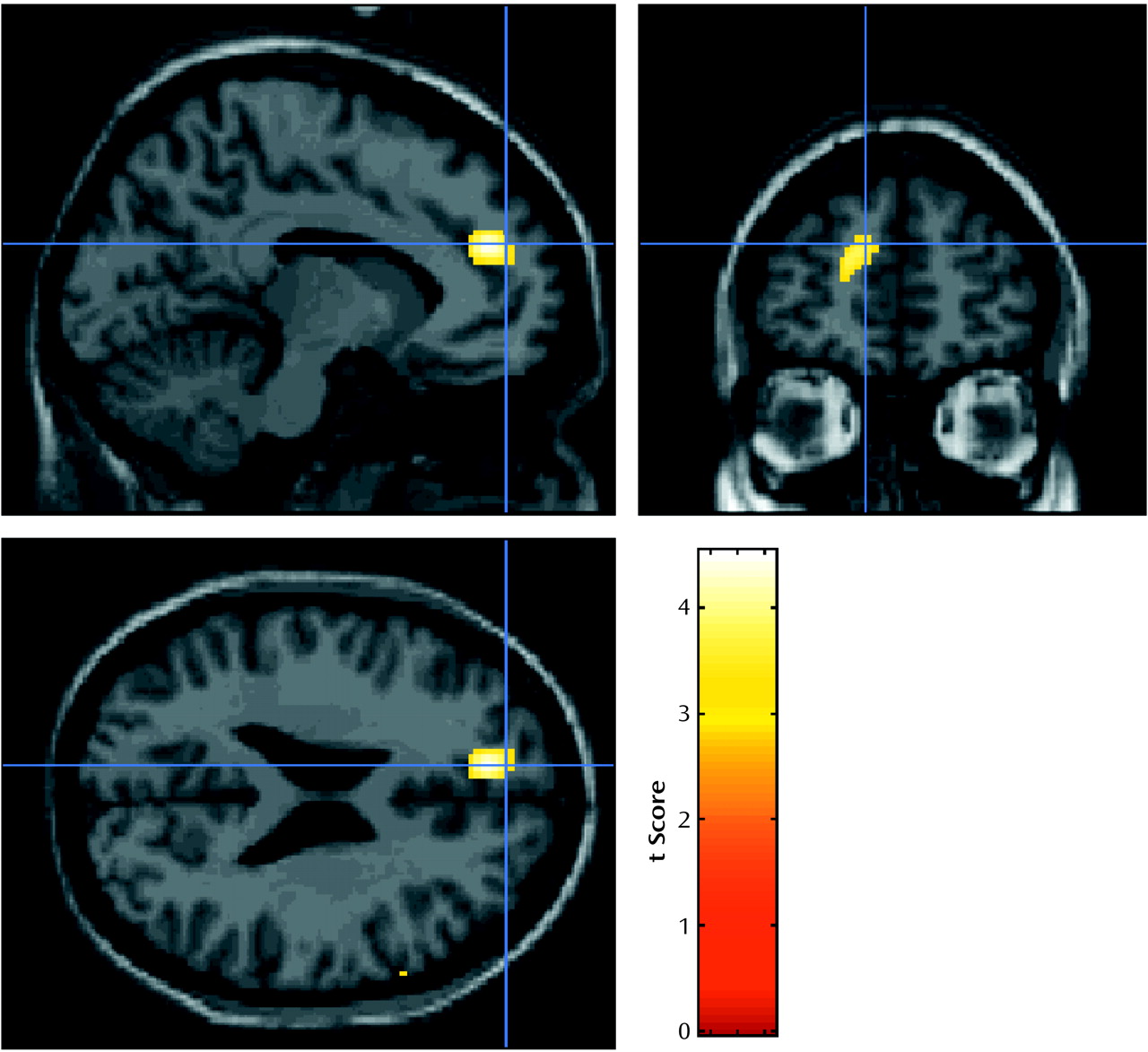
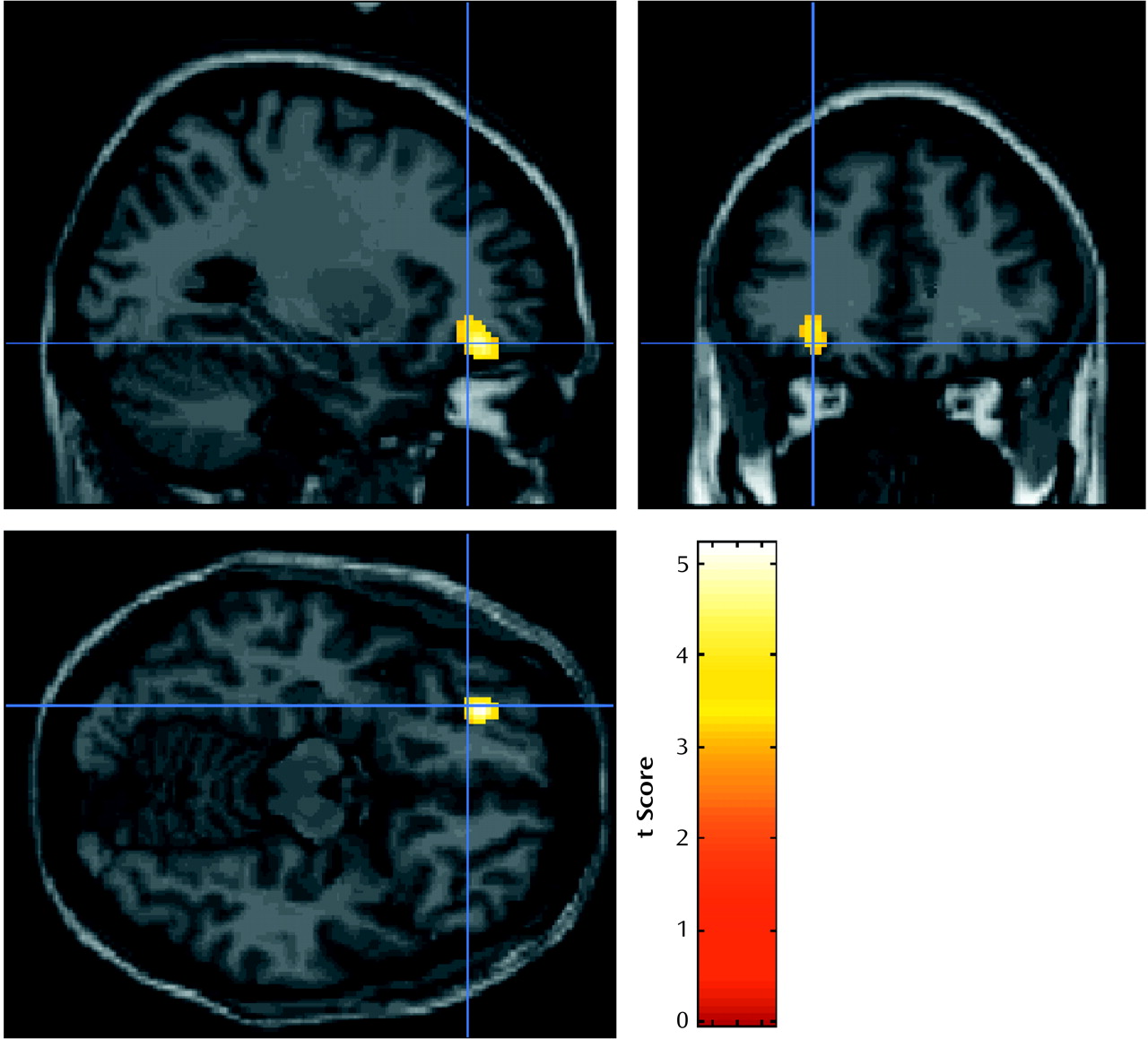
Two dissociable activation patterns of regional cerebral activation were observed in this study. The orbitofrontal cortex, which showed activation that correlated with the crave/urge scale score, did not show a significant activation with opiate-related cues, and similarly the left anterior cingulate/medial prefrontal region, which was activated by opiate-related stimuli, did not show activation that correlated significantly with the crave/urge scale score. Because these prefrontal foci do not overlap spatially and were identified in fundamentally different analyses, these results suggest that brain responses to drug-related stimuli per se may invoke different brain circuits from those involved in the craving induced by these stimuli.
We could use different methods of analysis to tease apart this distinction because craving induced by the drug-related stimuli carried over into the neutral scans. Specifically, the cognitive subtraction will be maximally sensitive, and a correlational analysis will be relatively insensitive, to detecting responses to the drug-related stimuli that do not show a carryover effect into the next scan. Conversely, areas of activation that show a slower response and particularly are slow to wear off will not be modeled well by the on/off model used for the condition comparison, but a correlational analysis will be maximally sensitive to this pattern.
The increase in rCBF in the left anterior cingulate/medial prefrontal region was consistent across all the subjects, even those who reported no opiate craving, and the response in the anterior cingulate was related to the duration of abstinence. Anterior cingulate activations are commonly observed in functional mapping studies, with a broad distinction being drawn between activation in caudal “cognitive” areas and rostral “affective” regions
(15). In our study, the focus of activation clearly lies within the latter region, and thus the activation is more likely to represent a neural response to the emotionally laden nature of the drug-related stimuli than to represent attentional allocation per se to the auditory stimuli. Activation of the anterior cingulate region in response to drug-related video stimuli has been shown to be modulated by activity in the midbrain in response to heroin administration in opiate-dependent subjects
(16). This activation was inferior to the activation reported here but remained within the “affective” region. Activation of the anterior cingulate has been reported with cue-induced cocaine craving
(7). However, it is not possible from the published data to further localize the effect.
Activation in this region, as measured by single photon emission computed tomography, has in addition been shown to be inversely correlated with the severity of naltrexone-precipitated opiate withdrawal
(17). We observed that several of our patients experienced conditioned withdrawal (e.g., sweating and piloerection) while listening to their craving experiences, although we did not measure these responses. Therefore, an alternative explanation is that the activation of the anterior cingulate, an area that was previously associated with withdrawal and pain, was a manifestation of conditioned withdrawal during the craving manipulation in our patients. However, if this explanation were correct, it would be expected that the difference in responses between the opiate-related and the neutral conditions would decrease as the duration of abstinence increased. In contrast, we found that this difference increased as duration of abstinence increased.
In addition, it could be theorized that exposure to stimuli that provoke craving for opiates should become a less familiar experience as the period of abstinence increases. Therefore, any habituation to such stimuli should gradually be lost, and, thus, they would be more arousing or alerting when encountered. Consequently, it would not be surprising that regions involved in emotional arousal and attention, such as the anterior cingulate/medial prefrontal cortex, would show increased relative activation to opiate-related stimuli with increasing length of abstinence.
Activation in the left orbitofrontal cortex showed a positive association with the composite crave/urge scale. This association of rCBF activation was present in all eight subjects who reported a craving response to the stimulus. Activation in this area might be more directly related to the subjective experience of craving rather than the early processing of the opiate-related stimuli. Other researchers have reported a similar significant correlation between urge to use heroin and rCBF in an adjacent area of the left inferior frontal cortex and an area of the orbitofrontal cortex in the opposite, right hemisphere
(11). Cocaine craving also correlates with orbitofrontal cortex activation, more medially when precipitated by withdrawal
(18) and in the right orbitofrontal cortex when induced by methylphenidate
(9). Bilateral orbitofrontal cortex activations are also observed in response to stimuli that elicit cocaine craving
(6,
10).
The data we reported here add to the emerging evidence, from studies using nonimaging techniques, that the orbitofrontal cortex plays an important role in drug dependence
(19,
20). The orbitofrontal cortex is linked to the mesolimbic dopamine system that mediates reward
(21) and to the subcallosal region of the anterior cingulate
(19,
20,
22) and is connected to the amygdala. In addition, the orbitofrontal cortex receives inputs from various sensory association areas and consequently plays an important role in expectancy and the reinforcing salience of stimuli
(23–
25). London et al.
(26) have suggested that the orbitofrontal cortex, through its connection with the amygdala, may play a role in evaluating the motivational value of stimuli and labeling the emotional experience of craving. This hypothesis could explain why activation in this region covaried with subjective craving measures in our study. Lastly, Volkow and Fowler
(27) have emphasized the role of the orbitofrontal cortex as part of the striatothalamic-orbitofrontal cortex circuit. Dysfunction in this region is associated with compulsive behavior and heightened motivation to access a drug of abuse
(27).
The limitations of our study include the lack of comparison subjects and a random effects statistical design. Thus, our conclusions are limited to this particular group of subjects. Only 67% of the subjects reported craving in response to the drug-related stimulus, although this low rate would not be unusual in the artificial environment of a brain scanner. A weakness of our initial categorical design was the carryover effect of craving into the neutral condition. However, we were able to take advantage of this effect by performing a correlational analysis in those subjects who craved to show a different pattern of activation.
The relationship of the visual analogue scale reports of subjective craving to real behavioral intentions is also open to some question, as none of the subjects acted on this craving by relapsing to heroin use. However, the majority of them were seeking help with craving as part of their ongoing treatment, and so the experience of craving was well known to them. The gradual habituation to the craving stimuli, shown by the visual analogue scale scores, in some of the subjects may also have increased the variance of responses, reducing the sensitivity of the cognitive subtraction analysis.
There were minor significant differences in the vividness, anxiety, and sadness visual analogue scales between the two conditions, but it is unlikely that these were responsible for the activations reported, as no significant rCBF changes correlated with scores on these scales (data not shown). Finally, movement by subjects is a possible source of artifactual changes in rCBF. However, for the changes reported here, this explanation is unlikely, because the areas of rCBF change that correlated with subject movement in a separate analysis did not overlap with any of the reported results (data not shown).
In conclusion, we have shown in this study that it is possible to induce opiate craving in a scanner environment and to map the neural correlates of craving and urge to use to activation of the orbitofrontal cortex. This neural response could be distinguished from activations in the left anterior cingulate/medial prefrontal region after exposure to drug-related stimuli.