Posttraumatic stress disorder (PTSD) develops in response to a traumatic event and describes a constellation of symptoms and behaviors that includes persistent reexperiencing of the trauma, avoidance of reminders of the trauma, numbing of positive emotions and social withdrawal, and symptoms of increased autonomic arousal. Epidemiological studies of PTSD in the United States
(1,
2) have estimated the lifetime prevalence to be between 8% and 12%. Approximately 10%–50% of the survivors of severe trauma will develop chronic PTSD, which often persists for years if untreated
(1–
3). Comorbid psychiatric disorders are extremely common: In the National Comorbidity Survey, approximately 80% of the individuals with PTSD also met criteria for at least one other DSM-III-R disorder
(1). PTSD is one of the most pervasively disabling of the affective and anxiety disorders and causes significant impairment in occupational and social functioning
(2–
4), higher rates of suicidality
(2,
5), and more medical illness
(6).
Investigations of the biology of PTSD have found alterations in the hypothalamic-pituitary-adrenocortical (HPA) axis and in catecholaminergic and serotonergic systems
(7–
10). The release of corticotropin releasing factor (CRF) from the median eminence of the hypothalamus appears to be modulated by serotonergic input from the midbrain raphe nuclei
(11). Evidence for the role of serotonin in the modulation of the HPA axis by means of modulation of CRF release comes from animal studies showing that long-term administration of selective serotonin reuptake inhibitors (SSRIs) reduces expression of paraventricular nucleus CRF mRNA
(12,
13). The SSRI paroxetine was found to reverse CRF alterations induced by stress associated with early maternal separation in primates
(14).
Given the evidence for biological alterations in PTSD, it is surprising that there have been few controlled pharmacotherapy trials. Early placebo-controlled drug studies found modest efficacy for the tricyclic antidepressants imipramine and amitriptyline and moderate efficacy for the monoamine oxidase inhibitor phenelzine
(15,
16). Van der Kolk et al.
(17) found that, among subjects with non-combat-related PTSD, treatment with the SSRI fluoxetine produced a 40% reduction in the severity of PTSD symptoms after 5 weeks compared to 15% with placebo. In a 12-week study, Connor et al.
(18) found fluoxetine treatment to be significantly more effective than placebo: 85% of the patients taking fluoxetine and 62% of the patients receiving placebo were rated “very much improved” or “much improved.” Finally, Brady et al.
(19) reported the results of a 12-week study in which sertraline was superior to placebo in reducing PTSD symptoms (53% versus 32%, respectively). Response was defined as a global rating of “very much improved” or “much improved” and a 30% reduction in PTSD symptom ratings.
As the manifestations of PTSD encompass an array of clinically distinct psychological and behavioral symptoms (reexperiencing, avoidance/numbing, and hyperarousal), most studies have examined treatment efficacy for each of these symptom domains. In the study by Van der Kolk et al.
(17), there was no significant improvement in intrusive or avoidance symptoms, but fluoxetine did produce significant improvement in hyperarousal and numbing symptoms. However, this study may have been too brief or of insufficient power to detect differences within individual clusters. Connor et al.
(18) did not report treatment response by symptom category in their fluoxetine trial; Brady et al.
(19) found sertraline significantly more effective than placebo in reducing symptoms of avoidance/numbing and hyperarousal but not reexperiencing. Thus, we know of no published drug trial to date that has demonstrated the efficacy of an SSRI in improving all three major symptom domains of PTSD.
Paroxetine was initially studied for the treatment of PTSD on the basis of findings from depression studies that suggested there were antianxiety effects for this SSRI
(20). A 12-week open trial showed that 11 (65%) of 17 patients with PTSD were rated as “very much improved” or “much improved” on the improvement scale of the Clinical Global Impression (CGI) scale and had a 48% reduction in mean scores for PTSD symptoms
(21). On the basis of this evidence, the present placebo-controlled, multicenter trial was conducted to test the efficacy and tolerability of paroxetine in the treatment of PTSD.
Method
Study Design
This study was a 12-week, double-blind comparison of 20 mg/day and 40 mg/day of paroxetine and of placebo in adults with chronic PTSD. Eligible patients entered a 1-week placebo run-in period to evaluate compliance with study procedures, followed by random assignment to one of the three treatments. All paroxetine-treated patients started therapy at 20 mg/day; patients assigned to the 40-mg/day group then received 30 mg/day during week 2 and 40 mg/day from the beginning of week 3. Assessments were conducted at weeks 1, 2, 4, 6, 8, and 12. Chloral hydrate was permitted at doses up to 1000 mg per night for sleep disturbance only during the placebo run-in period and week 1.
Patient Selection
Study subjects were male and female outpatients 18 years or older who met DSM-IV criteria for chronic PTSD as determined by the diagnostic version of the Clinician-Administered PTSD Scale, part 1
(22), and the Mini International Neuropsychiatric Interview
(23). The Clinician-Administered PTSD Scale is a validated clinical interview designed to assess the frequency and severity of each of the 17 DSM-IV-defined PTSD symptoms as well as criterion F (social and occupational impairment). The Clinician-Administered PTSD Scale, part 1, is used to assess a patient’s current and lifetime DSM-IV diagnosis of PTSD, while the Clinician-Administered PTSD Scale, part 2, is used to evaluate symptom severity and change.
Concurrent affective and anxiety disorders were allowed in study patients, provided that PTSD was considered the principal diagnosis (i.e., the main focus of attention or need for treatment) and the onset of PTSD preceded that of concurrent disorders. Furthermore, patients could not have had another axis I disorder as a principal diagnosis within 6 months of screening. A Clinician-Administered PTSD Scale, part 2, total score of 50 points or higher was required for study entry. For female patients of childbearing potential, participation was contingent on a negative serum pregnancy test and a medically accepted method of contraception. Other exclusion criteria were 1) receiving disability payments or involvement in litigation related to PTSD or any other psychiatric illness, 2) alcohol or substance abuse or dependence within 6 months of screening, 3) taking psychotropic medications within 2 weeks of the first dose of study medication (or 4 weeks for fluoxetine), 4) having psychotherapy or ECT within 12 weeks of screening, 5) being a homicidal or suicidal risk, 6) intolerance to paroxetine or any other SSRI, or 7) having a serious medical condition.
The study was conducted at 59 centers in the United States. At each center, the study protocol was approved by an institutional review board, and written informed consent was obtained from all subjects before initiation of study procedures.
Outcome Measures
The primary outcome measures were change in total score from baseline to the week-12 endpoint on the Clinician-Administered PTSD Scale, part 2, and the proportion of responders with a CGI improvement rating of “very much improved” or “much improved.” Secondary outcome measures included change in total scores from baseline to endpoint on the patient-rated Davidson Trauma Scale
(24) and the clinician-rated Treatment Outcome PTSD Scale
(25). In order to evaluate improvement in the three symptom domains of PTSD, change in scores from baseline to endpoint was examined on the Clinician-Administered PTSD Scale, part 2, and on the Davidson Trauma Scale symptom clusters (reexperiencing, avoidance/numbing, and hyperarousal). Functional impairment was assessed with the Sheehan Disability Scale
(26) and items 22 (social functioning) and 23 (occupational or important role functioning) of the Clinician-Administered PTSD Scale, part 2. The total score on the clinician-rated Montgomery-Åsberg Depression Rating Scale
(27) was used to evaluate comorbid depressive symptoms.
Safety assessments conducted at screening and endpoint or at study discontinuation included a complete physical examination, a laboratory evaluation (including clinical chemistry and hematology tests and a urinalysis), and an ECG. At each scheduled visit, the patient’s sitting heart rate and blood pressure were also documented. The time of illness onset, duration of illness, severity of illness, any action taken, and outcome of observed or spontaneously reported adverse experiences were recorded. The type, dose, reason for treatment, and duration of use of concomitant medications were also recorded at each visit.
Statistical Analyses
Statistical analyses were based on the data set with the last observation carried forward for the patients who were randomly assigned to groups and who had at least one treatment assessment (intent-to-treat group). All analyses were two-sided comparisons between the active treatments and placebo. Continuous variables were analyzed by using general linear models
(28), with adjustment for treatment center, gender of patient, type of trauma, time since trauma occurred, severity of baseline score, and depressive symptoms. Logistic regression was used to examine differences in the proportions of patients whose scores on the CGI improved (responders) among groups. The primary analyses of the change in total scores from baseline on Clinician-Administered PTSD Scale, part 2, and of the proportion of the responders (as per CGI rating) were adjusted for multiple comparisons by using Hochberg’s procedure
(29). Changes in scores from baseline on the social and occupational impairment items of the Clinician-Administered PTSD Scale, part 2, were analyzed by using Wilcoxon’s rank sum test
(30). Fisher’s exact test
(31) was used to test differences in the rates of study discontinuation caused by adverse events. Treatment-by-covariate interaction effects were considered statistically significant if p<0.10. All other statistical tests were considered statistically significant if p<0.05.
A post hoc analysis of the changes in scores on the Clinician-Administered PTSD Scale, part 2, in patients with and without major depressive disorder was conducted to examine the disorder-specific efficacy of paroxetine. A second post hoc analysis examined whether paroxetine was effective in both men and women.
Results
Characteristics and Baseline Severity
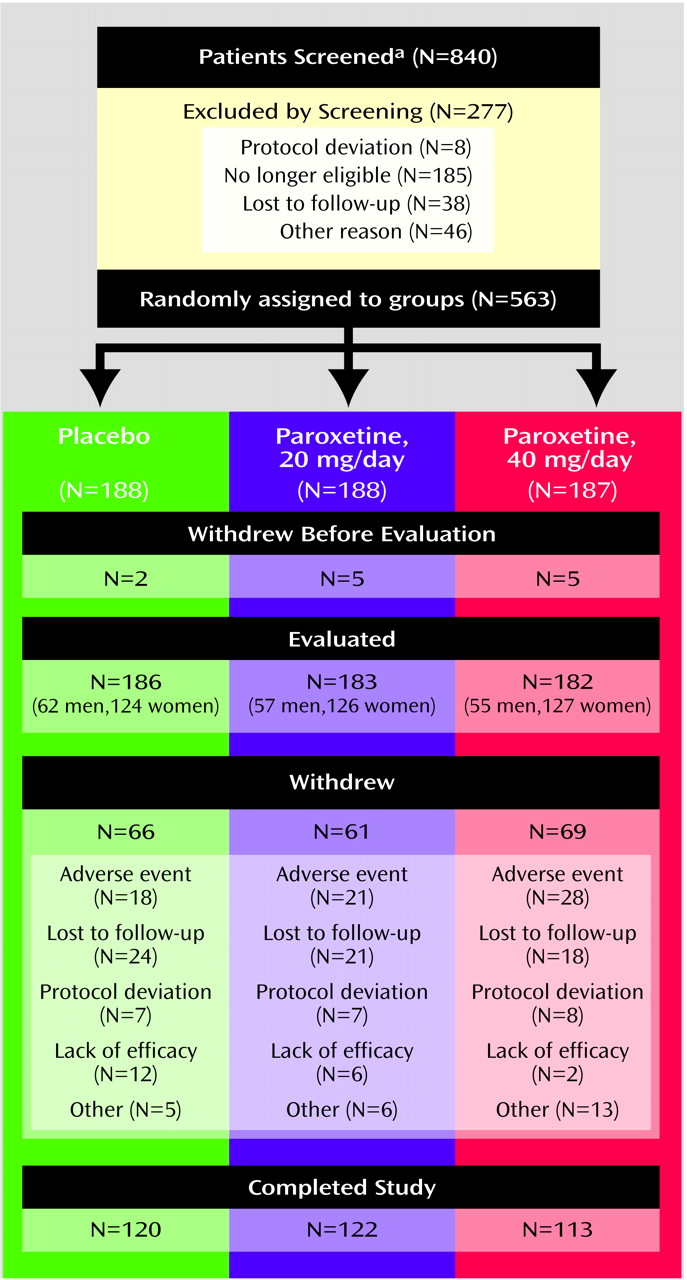
A total of 840 patients entered the screening/placebo run-in phase of the study. Of these, 277 patients were not randomly assigned to treatment groups at baseline for the reasons shown in
Figure 1. Of the 563 patients randomly assigned to receive treatment, 12 patients (two receiving placebo and five in each paroxetine dose group) were lost to follow-up before the first postbaseline assessment. The remaining 551 patients had at least one treatment assessment and comprised the intent-to-treat group for all analyses (186 receiving placebo, 183 receiving 20 mg/day of paroxetine, and 182 receiving 40 mg/day of paroxetine).
Figure 1 summarizes patient disposition throughout the study. Patient demographic characteristics did not differ across the three randomly assigned groups. As expected, there were about twice as many women as men in the patient group. The average age was 41.8 years (SD=11.6), and the majority (>90% in each group) were white. The mean Clinician-Administered PTSD Scale, part 2, total score at baseline was 74.4 (SD=15.9) in the placebo group and 75.3 (SD=16.1) and 74.3 (SD=15.6) in the 20-mg/day and 40-mg/day paroxetine groups, respectively.
The most common trauma types in the three treatment groups were physical or sexual assault (48%–54%), witnessing injury or death (17%–18%), serious accident or injury (6%–12%), and combat (5%–8%). Approximately 45% of the study group met DSM-IV criteria for major depressive disorder. The baseline mean score on the Montgomery-Åsberg Depression Rating Scale was 24.4 (SD=7.6) for the placebo group and 25.2 (SD=8.6) and 24.9 (SD=7.6) for the 20-mg/day and 40-mg/day paroxetine groups, respectively. Other comorbid diagnoses included generalized anxiety disorder (28%–32%), agoraphobia (21%–25%), panic disorder (14%–17%), and dysthymia (9%–12%). On average, the patients’ index trauma occurred 15.7 years (SD=14.8) before the study began. A total of 355 patients (64%) completed the 12-week study. Completion rates were similar across groups: 120 (65%) of the placebo group, 122 (67%) of the group receiving 20 mg/day of paroxetine, and 113 (62%) of the group receiving 40 mg/day of paroxetine. The most frequently reported reason for early withdrawal from the study was “lost to follow-up” among placebo-treated patients (13%) and adverse events among patients treated with 20 mg/day (11%) or 40 mg/day (15%) of paroxetine. The number of withdrawals due to an adverse event was not significantly different among the placebo (18 of 186) and 20-mg/day (21 of 183; p<0.62, Fisher’s exact test) and 40-mg/day (28 of 182; p<0.12, Fisher’s exact test) paroxetine treatment groups.
Primary and Secondary Outcomes
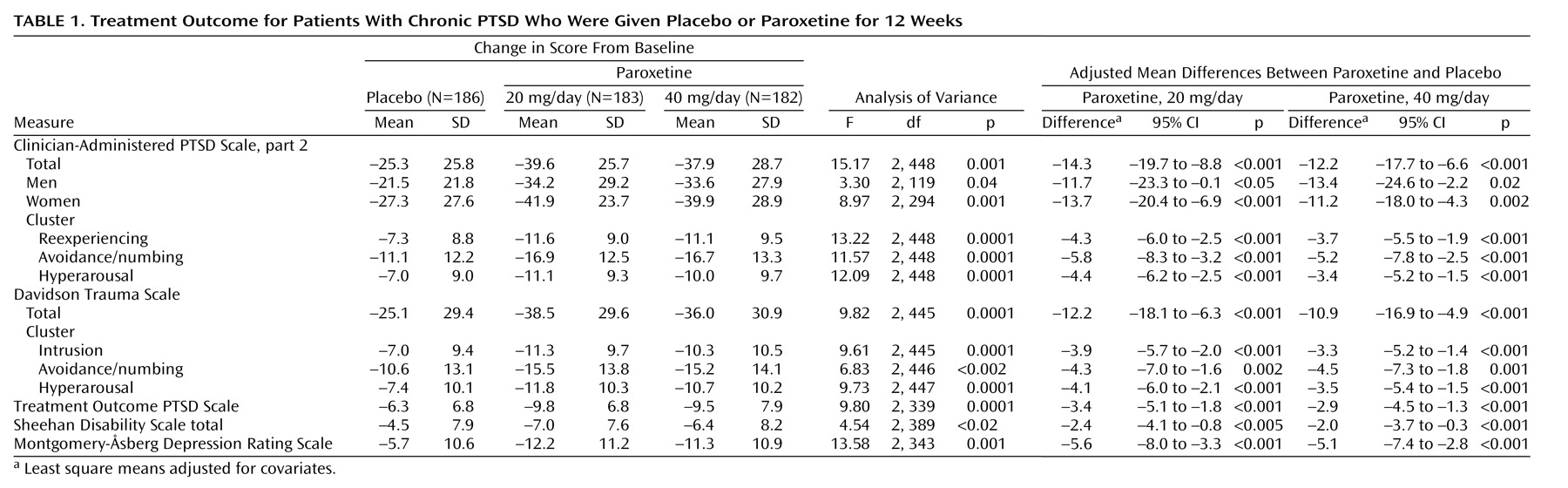
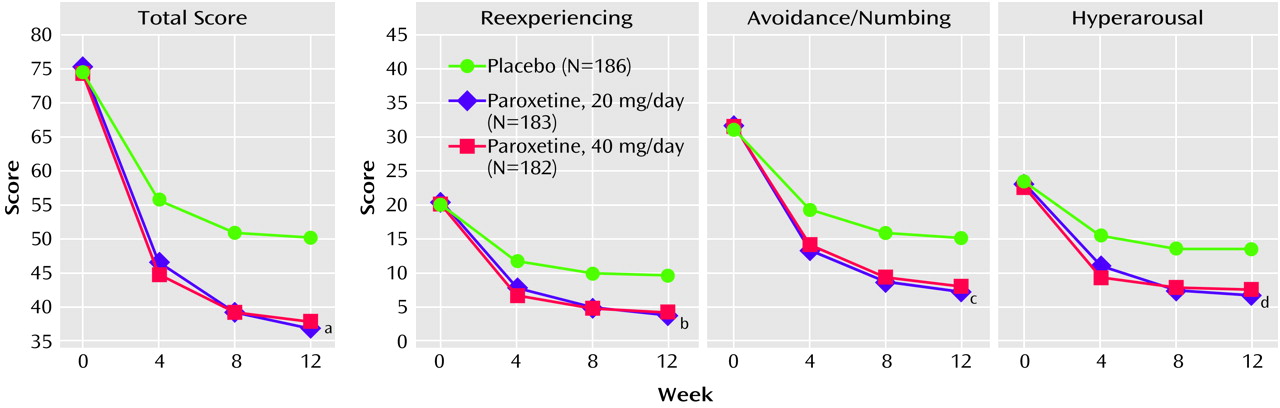
Table 1 presents for each treatment group the change in scores from baseline to endpoint and the covariate-adjusted differences among the treatments for the main outcome variables. Improvement was significantly greater for the paroxetine groups compared to the placebo group on all efficacy measures. Both groups receiving paroxetine had total scores that were significantly different from those of the placebo group on the Clinician-Administered PTSD Scale, part 2, at weeks 4, 8 and 12 (
Figure 2). Moreover, paroxetine had significantly greater effects than placebo at all time points on each of the three PTSD symptom cluster scores (
Figure 2; reexperiencing, avoidance/numbing, and hyperarousal) on the Clinician-Administered PTSD Scale, part 2 (data for weeks 4 and 8 available from Dr. Marshall).
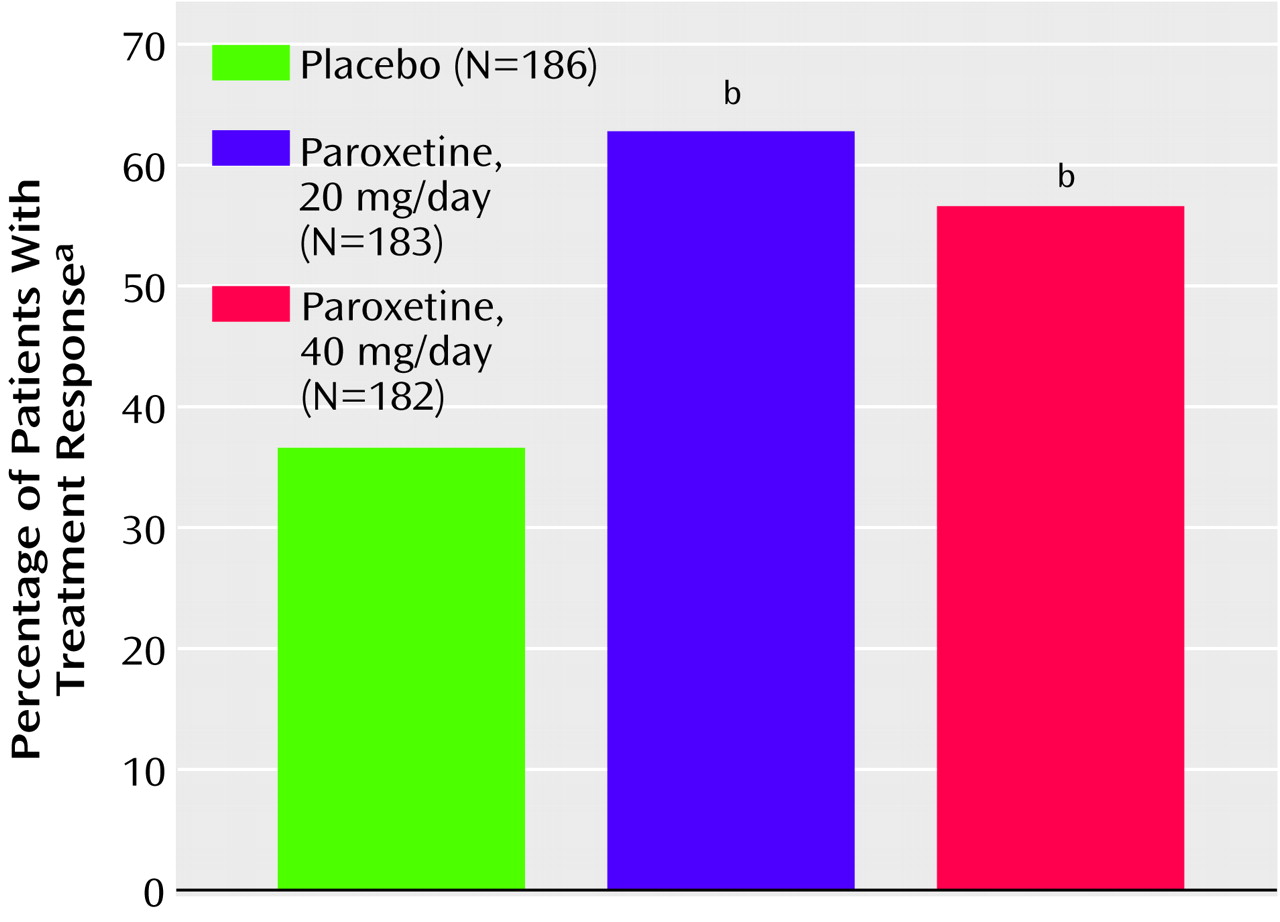
Compared to patients treated with placebo, significantly more patients treated with paroxetine at both doses were rated as responders on the CGI scale at endpoint (
Figure 3). Functional improvement, as assessed by the Sheehan Disability Scale, was also significantly greater for the paroxetine-treated patients compared to the placebo-treated patients at endpoint in the three component domains: work (20 mg/day of paroxetine versus placebo [t=–3.21, df=389, p=0.001]; 40 mg/day of paroxetine versus placebo [t=–1.64, df=389, p<0.11]), social life (20 mg/day of paroxetine versus placebo [t=–2.64, df=421, p=0.009]; 40 mg/day of paroxetine versus placebo [t=–2.26, df=421, p<0.03]), and family life (20 mg/day of paroxetine versus placebo [t=–2.24, df=421, p<0.05]; 40 mg/day of paroxetine versus placebo [t=–2.21, df=421, p<0.04]). Likewise, as assessed by the Clinician-Administered PTSD Scale, part 2, social and occupational impairment improved to a significantly greater degree in patients who received paroxetine than in those who received placebo (social impairment: 20 mg/day of paroxetine [N=166] and placebo [N=167], z=–4.06, p<0.001; 40 mg/day of paroxetine [N=156] and placebo [N=167], z=–3.67, p<0.001) (occupational impairment: 20 mg/day of paroxetine [N=166] and placebo [N=167], z=–2.21, p<0.03; 40 mg/day of paroxetine [N=156] and placebo [N=167], z=–2.70, p=0.007).
Patients With and Without Comorbid Depression
In our analysis of the change in total scores on the Clinician-Administered PTSD Scale, part 2, for the patient subgroups with and without a baseline diagnosis of major depressive disorder, paroxetine reduced PTSD symptoms to a significantly greater degree than placebo in both subgroups (nondepressed: F=12.33, df=2, 229, p<0.001; 20 mg/day of paroxetine and placebo: mean=–16.8-point change in score, 95% confidence interval [CI]=–23.7 to –9.8, p<0.001; 40 mg of paroxetine and placebo: mean=–12.7-point change in score, 95% CI=–19.8 to –5.6, p<0.001) (depressed: F=4.07, df=2, 181, p<0.02; 20 mg/day of paroxetine and placebo: mean=–11.0-point change in score, 95% CI=–20.4 to –1.7, p<0.03; 40 mg/day of paroxetine and placebo: mean=–11.8-point change in score, 95% CI=–20.9 to –2.7, p<0.02).
Treatment Response by Gender
Both male and female patients treated with paroxetine achieved similar and statistically significant drug-versus-placebo differences in total scores on the Clinician-Administered PTSD Scale, part 2, at endpoint compared to placebo-treated patients (
Table 1).
Tolerability
Paroxetine was well tolerated by the patients in this study. The most commonly reported adverse events associated with paroxetine use (with an incidence of at least 10% and twice that of placebo) were asthenia, diarrhea, abnormal ejaculation, impotence, nausea, and somnolence. The majority of the treatment-emergent adverse events were rated as mild to moderate in severity and most occurred at the beginning of treatment. There were no unexpected adverse events, and serious adverse experiences were infrequent (nine of the 365 patients treated with paroxetine). In seven of these patients, the adverse events were rated by the investigators as “unrelated or “probably unrelated” to paroxetine treatment. Two cases of adverse events were rated as “related” or “possibly related” to the study medication. In the first case, the patient took his daily dose of paroxetine twice for several days because he could not recall taking his dose in the morning. He reported experiencing no ill effects but was withdrawn from the study because of noncompliance. The second patient who experienced adverse events that were “related” to paroxetine treatment experienced an onset of severe headaches on day 2 of paroxetine treatment and discontinued participation in the study.
Subjects’ laboratory values, vital signs, and ECG results were generally similar across treatment groups. Changes in these values were minor, infrequent, and not considered clinically meaningful.
Discussion
The results of this placebo-controlled trial provide convincing evidence that paroxetine treatment at 20 and 40 mg/day is effective and well tolerated in the treatment of adult men and women with PTSD. Treatment with paroxetine resulted in response rates of 62% (N=113, 20-mg/day group) and 54% (N=99, 40-mg/day group), compared to approximately 37% (N=67) of those who were taking placebo (
Figure 3). Outcomes did not vary according to patients’ gender, trauma type, time since onset of trauma, or the severity of PTSD or depressive symptoms at baseline.
The symptom profile and chronic nature of this disorder frequently lead to substantial disability and impairment of an individual’s ability to function in major life spheres such as work and interpersonal relationships. It is noteworthy that given the disease chronicity in this patient group and the “moderately severe” to “severe” impairment observed at baseline (total score on the Sheehan Disability Scale
[26]: mean=16.5, SD=6.4), the paroxetine-treated patients experienced a reduction in their total mean score on the Sheehan Disability Scale of approximately 40% after a relatively short course of treatment. This result is comparable to that observed in subjects with social anxiety disorder
(32) and panic disorder
(33).
Similar to rates reported in the epidemiological literature
(1), approximately one-half of the patients in this study had comorbid major depressive disorder. An important question is whether comorbidity influences treatment response. We found that paroxetine was superior to placebo in the treatment of PTSD in patients with and without comorbid major depressive disorder. As expected, paroxetine was also significantly better than placebo in reducing the severity of depressive symptoms on the Montgomery-Åsberg Depression Rating Scale.
Paroxetine was found to be safe and well tolerated. The dropout rate due to adverse events and the adverse events observed are comparable to those reported for paroxetine in the treatment of other anxiety disorders
(32–
35). The results of this study do not indicate a dose/response relationship on the primary measures of efficacy, which suggests that 20 mg/day of paroxetine may be an effective dose for the majority of PTSD patients. However, in clinical practice, a higher dose of paroxetine may be useful in some cases, and appropriate treatment should include adjustment of dose on the basis of patient response and tolerability.
One limitation of the present study is that it did not explore long-term treatment. Because of the chronic and severely debilitating nature of PTSD, an adequate course of pharmacotherapy is likely to be longer than 12 weeks. Further studies are therefore required to establish the optimal treatment duration for PTSD and to evaluate the effectiveness of long-term treatment on the symptoms of comorbid disorders.
This study is the first to our knowledge to demonstrate that an SSRI effectively ameliorates each of the major symptom clusters of PTSD (reexperiencing, avoidance/numbing, and hyperarousal). Statistically significant treatment benefits were evident at week 4, and improvement continued through week 12. Previous SSRI trials
(17,
19) found efficacy in treating the avoidance and hyperarousal symptoms of PTSD but not reexperiencing. This study also addressed the important question of whether medication response is different in men and women with chronic PTSD because rates of trauma vary between men and women in the community as do the types of trauma they typically experience. To date, this issue has not been adequately addressed owing to small group sizes and/or differences in types of trauma between men and women in clinical trials
(19,
36). We found paroxetine to be effective in both men and women with chronic PTSD.
From a practical standpoint, however, as in all other clinical trials of a single treatment for chronic PTSD, a significant proportion of patients improved but did not fully recover. This observation holds across both medication and psychotherapy trials
(37). For example, although therapy involving prolonged exposure has been shown to be highly effective in treating chronic PTSD
(38), the treatment appears limited in its applicability to a subset of patients because of symptom exacerbation, an inability to engage in the treatment, and other factors
(39). Thus, for some patients, a prior trial of medication might allow more effective engagement in the psychotherapy process. In addition, future research should investigate the combination of psychotherapeutic approaches and pharmacotherapy as a strategy for maximizing treatment outcome for adults with chronic PTSD. Finally, because the majority of patients in this trial were chronic sufferers of PTSD, it is possible that treatment response in many cases was affected by the chronicity of the disorder. Future studies might therefore be directed toward early intervention as a strategy for achieving the better response than has been observed in other treatments for chronic PTSD.