The comorbid presentation of cannabis abuse and depression is relatively common in clinical and community populations
(1,
2). However, the degree to which psychiatric disorders such as depression are predisposing risk factors for substance abuse, or vice versa, is a subject of controversy
(3,
4). On the one hand, individuals may use cannabis to self-medicate their dysphoria. On the other hand, chronic cannabis use may exacerbate, if not induce, dysphoria. Policies regarding the medical and legal status of cannabis may be better informed by a more definitive estimation of the risks posed by cannabis abuse, such as whether it is associated with a greater risk of depression.
Anecdotal studies of clinical populations suggest that individuals who abuse cannabis are self-medicating their anxiety and depression
(5,
6). Similarly, cross-sectional studies of nonclinical populations suggest that cannabis use follows depression and that impaired motivation is a manifestation of depression rather than a consequence of cannabis use, although the dose-response association of cannabis use and depression has not been consistently reported
(5–
7). However, anecdotal and cross-sectional studies rely on retrospective reports of depressive symptoms, which are often biased by the reconstruction of memories based on current affective states, particularly in depressed individuals
(8,
9). In contrast to cross-sectional studies, longitudinal studies of adult community populations have found an increase in depressive symptoms after cannabis use, although these studies have not controlled for the baseline comorbidity of cannabis use and depression
(10–
12). Longitudinal studies of children and adolescents have found that depression increases the risk of later cannabis use, rather than vice versa, but not consistently across populations
(13,
14).
Overall, the literature on the comorbidity of cannabis abuse and depression is divided. Longitudinal studies suggest that cannabis abuse in adults increases depressive symptoms, whereas cross-sectional studies suggest that a history of depression explains the dysphoria associated with cannabis abuse. The literature leaves open to question if cannabis abuse increases the incidence (i.e., new cases) of depression or if depression increases the incidence of cannabis abuse. Thus, the etiological significance of depression and cannabis abuse as risk factors for cannabis abuse and depression, respectively, is not well understood. The current research addresses these issues through a longitudinal study of a randomly sampled adult population over a nearly 15-year period. Consistent with longitudinal studies and contrary to cross-sectional studies, depressive symptoms were expected to follow cannabis abuse, rather than vice versa.
Results
Attrition
The participants who could not be located at the time of the follow-up assessment tended to be older and less educated, with lower household incomes, more physical illness, and fewer baseline symptoms of depression, mania, and somatization. These results are consistent with those from a previous report on the ECA follow-up sample
(19). In addition, participants lost to follow-up tended to be unmarried compared with those retained (χ
2=14.9, df=1, p<0.01) and had a lower prevalence of cannabis abuse than did those retained (2.6% versus 4.3%, respectively; χ
2=6.6, df=1, p<0.05).
Baseline Cannabis Abuse and Depression
Of the 1,920 subjects retained, the 83 participants with a baseline cannabis abuse diagnosis reported more baseline depressive symptoms than the 1,837 without a cannabis abuse diagnosis (F=36.3, df=1, 1918, p<0.0001). In the 83 participants with a baseline diagnosis of cannabis abuse, differences between those with and without depressive symptoms were examined. The 15 who reported no depressive symptoms were not significantly different from the 68 who did report depressive symptoms, except that the latter group had more symptoms of mania (F=5.7, df=1, 81, p<0.04) and somatization (F=6.7, df=1, 81, p<0.05). Thus, the cohort of 15 cannabis abusers followed for assessment of depression risk was a representative sample of cannabis abusers in this population at baseline.
Risk of Depressive Symptoms at Follow-Up Assessment
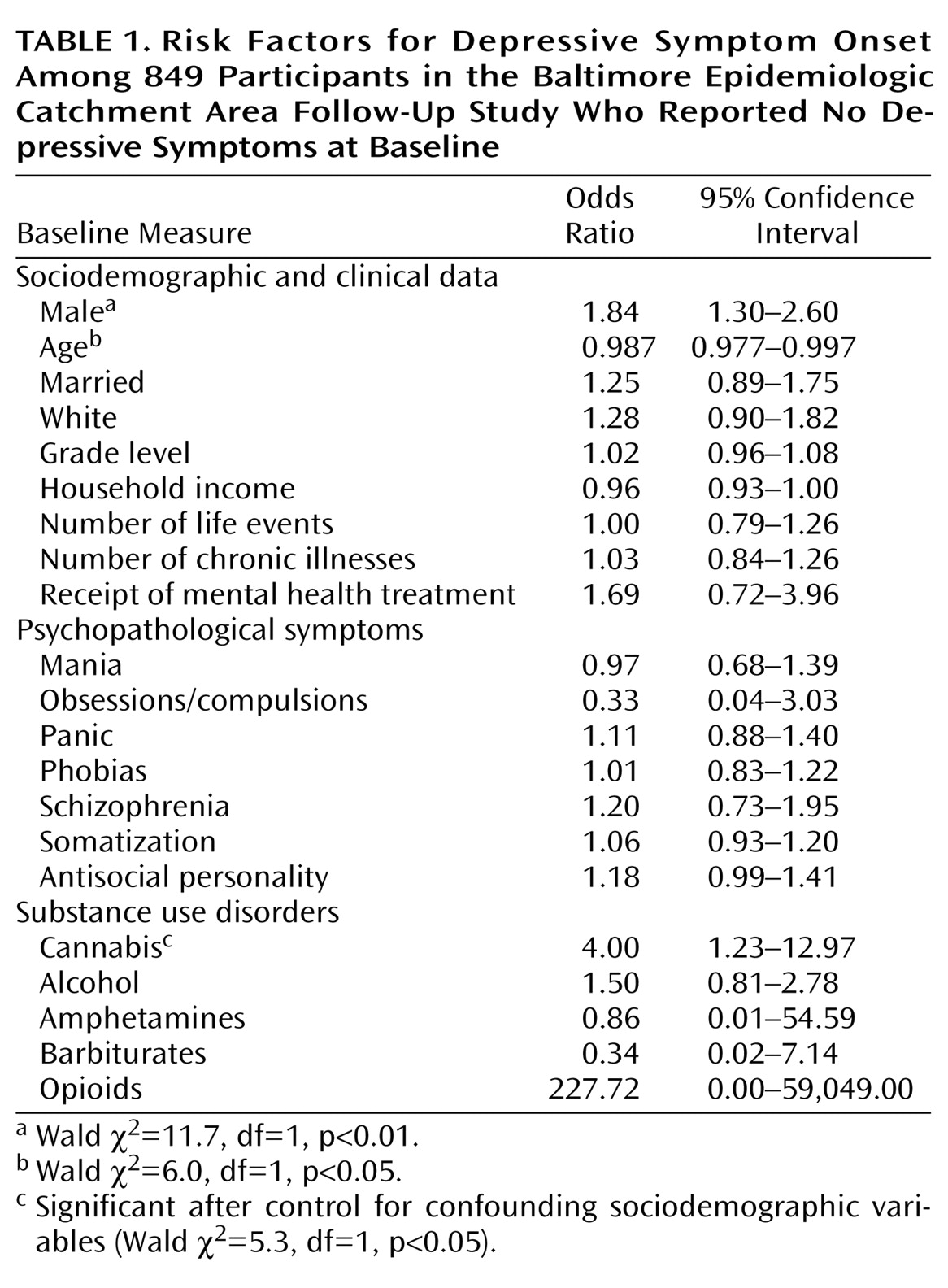
The risk of the onset of depressive symptoms during the follow-up period in participants diagnosed with cannabis abuse at baseline was about four times greater than the risk in participants without a baseline cannabis abuse diagnosis (odds ratio=4.49, 95% confidence interval [CI]=1.51–13.26; Wald χ
2=7.4, df=1, p<0.01). This association was little changed after adjusting for the association of baseline depressive symptoms with other possibly confounding covariates. The risk of depressive symptoms remained about four times greater in participants diagnosed with cannabis abuse at baseline than in those without a baseline cannabis abuse diagnosis (
Table 1).
Of the 267 participants who experienced depressive symptoms during the follow-up period, 3.7% (N=10) had been diagnosed with cannabis abuse at baseline, whereas only 0.9% (N=5) of the 582 participants who did not report depressive symptoms had a baseline cannabis abuse diagnosis. A significant association of cannabis abuse at baseline and depressive symptoms at the follow-up assessment was found (χ2=8.0, df=1, p<0.01). Fisher’s exact test was performed to test the statistical significance of this association, since the expected count of participants with both a baseline cannabis abuse diagnosis and depressive symptoms at the follow-up assessment was less than five. After this test (either one-tailed or two-tailed), the association remained significant (p<0.01).
Risk of Cannabis Abuse Symptoms at Follow-Up Assessment
In the 1,837 participants with no diagnosis of cannabis abuse at baseline, the occurrence of any cannabis abuse symptoms during the follow-up period was not significantly predicted by baseline depressive symptoms, either with or without adjustment for baseline covariates. The significant baseline covariates for cannabis abuse at the follow-up assessment included amphetamine abuse (odds ratio=7.77, 95% CI=1.82–33.07; Wald χ2=7.7, df=1, p<0.01), opioid abuse (odds ratio=10.46, 95% CI=1.43–76.16; Wald χ2=5.4, df=1, p<0.05), female gender (odds ratio=0.32, 95% CI=0.19–0.53; Wald χ2=20.7, df=1, p<0.001), and age (odds ratio=0.88, 95% CI=0.85–0.91; Wald χ2=62.9, df=1, p<0.001). None of the other covariates entered in the equation was significant.
Post Hoc Analyses
A post hoc analysis was performed to identify the particular symptoms of depression predicted by cannabis abuse. The occurrence of each of the nine depressive symptoms during the follow-up period was predicted by using the same baseline measures used to predict the incidence of any of the symptoms. Only suicidal ideation and anhedonia during the follow-up period were predicted by a baseline diagnosis of cannabis abuse. Of the 98 participants with anhedonia, 35 also had suicidal ideation (χ2=64.6, df=1, p<0.001).
The incidence of suicidal ideation was approximately four times greater in cannabis abusers than in nonabusers (odds ratio=4.55, 95% CI=1.37–15.12; Wald χ2=6.1, df=1, p<0.05) after adjustment for baseline covariates. The significant baseline covariates of suicidal ideation during the follow-up period included female gender (odds ratio=1.90, 95% CI=1.11–3.24; Wald χ2=5.6, df=1, p<0.05), age (odds ratio=0.98, 95% CI=0.95–0.98; Wald χ2=20.1, df=1, p<0.05), and white race (odds ratio=1.86, 95% CI=1.09–3.15; Wald χ2=5.2, df=1, p<0.05). None of the other covariates entered into the equation was significant. Of the 97 participants with thoughts about death during the follow-up period, six (6.2%) had a cannabis abuse diagnosis at baseline, whereas nine (1.2%) of the 752 participants without thoughts of death had a baseline diagnosis of cannabis abuse (χ2=12.3, df=1, p<0.01). Fisher’s exact test found this association to be significant (p<0.01).
Similarly, the incidence of anhedonia was approximately four times greater in cannabis abusers than in nonabusers (odds ratio=4.32, 95% CI=1.32–14.16; Wald χ2=5.8, df=1, p<0.05) after adjustment for the baseline covariates entered into the equation. The significant baseline covariates of anhedonia during the follow-up period were age (odds ratio=0.97, 95% CI=0.96–0.99; Wald χ2=12.4, df=1, p<0.0001) and antisocial symptoms (odds ratio=1.42, 95% CI=1.16–1.75; Wald χ2=11.1, df=1, p<0.001). None of the other covariates entered into the equation was significant. Of the 98 participants who experienced anhedonia during the follow-up period, six (6.1%) had a baseline diagnosis of cannabis abuse, whereas nine (1.2%) of the 751 participants who did not experience anhedonia had a baseline diagnosis of cannabis abuse (χ2=12.1, df=1, p<0.01). Fisher’s exact test found this association to be significant (p<0.01).
Discussion
The increase in incidence of depressive symptoms in individuals who abused cannabis cannot be explained by an underreporting of baseline depressive symptoms, given that they were more likely to report depressive symptoms than individuals who did not abuse cannabis, as reported here and in many other studies
(5–
9). The risk of depression found here also cannot be explained by factors confounded with cannabis abuse, which were ruled out as superior predictors of depressive symptoms (e.g., alcohol abuse, age, stressful life events). Nonetheless, confounding characteristics not assessed at baseline, and therefore not available for analysis, may account for the greater risk of depressive symptoms (e.g., suicidal ideation and anhedonia) in adults diagnosed with cannabis abuse. Further research is required to identify other characteristics of individuals who abuse cannabis that may explain their greater risk of subsequent depressive symptoms.
The inability to locate individuals with depressive and cannabis abuse symptoms during the follow-up period may have resulted in an underestimation of their association. Bias in the estimation of this association would have been introduced if the retention of individuals with one set of symptoms was greater than the retention of individuals with the other set of symptoms, which was not the case. Selective retention of individuals with certain characteristics assessed here is not likely to have biased the estimates, since these characteristics failed to confound the estimated associations. Although the estimated associations are based on small numbers of individuals and represent small effect sizes, the results may nonetheless generalize to large numbers of people in the general population, and the small effect sizes may nonetheless have great
practical validity (20).
The results underscore the importance of cannabis abuse prevention rather than treatment because they address the incidence (i.e., new cases) of depressive symptoms rather than the prevalence of depressive disorder. This finding extends those of the national ECA study
(1), which found that alcohol abuse and cocaine, but not cannabis or other drugs, were risk factors for suicide over the initial 1–2-year period of the study (1980–1981).
Obviously, causal associations or other mechanisms that explain the higher risk of depression in cannabis abusers cannot be determined by an epidemiological survey. However, a physiological mechanism underlying a causal association of cannabis use and suicide has been proposed, although it is somewhat limited to young male subjects
(21). Cannabis increases levels of interferon-gamma, which inhibits the activity of aromatase, the enzyme that converts androgens to estrogen. This in turn creates a deficiency in estrogen, a hormone that augments the synthesis of serotonin, thereby creating a deficiency in serotonin characteristic of major depression and suicide. Further research with animal models of cannabis-induced depression may be warranted.
The finding here that baseline depressive symptoms failed to predict subsequent cannabis abuse symptoms is not inconsistent with other research that has found that antidepressant medications reduce cannabis use in clinical populations of adult alcoholics
(22). Treating depression may reduce cannabis use in clinical populations, but depression did not necessarily lead these individuals to initiate cannabis use. Further, the results of the current research do not necessarily have implications for public policy regarding the decriminalization of cannabis. A reduction in cannabis abuse, as opposed to its prevention, will not necessarily reduce levels of dysphoria in the general population. Also, such a reduction would not necessarily impact public health or health service utilization, given that the level of dysphoria observed was subclinical. Subclinical levels of anhedonia in cannabis abusers may constitute an “amotivational” syndrome, but the degree to which such an amotivational syndrome impairs social or occupational functioning cannot be ascertained here and requires further study. Nonetheless, such subclinical dysphoria could represent a preclinical condition, particularly in certain subpopulations.
Finally, the results of this study do not have obvious implications for policies regarding the prescription of cannabis for medical conditions. The creation of depression as a result of prescribed cannabis use cannot be ascertained on the basis of the current study, which examined the self-administration of excessive levels of cannabis from illicit sources in an uncontrolled context. Thus, the higher suicide risk associated with cannabis abuse will not necessarily occur in cases in which cannabis is medically prescribed, even if patients have terminal illnesses or other disorders that also increase their suicide risk. Despite the limitations of the current study, the results suggest that the potentially serious consequences of cannabis abuse require further research.