At present, optimal pharmacologic treatment leaves many schizophrenia patients with residual symptoms and cognitive impairment
(1). Although augmentation of antipsychotic agents is often attempted, no single strategy demonstrates clear superiority. Omega-3 fatty acids are potentially attractive augmentation agents because of their favorable side effect profile and potential to restore disturbances in the metabolism of synaptic membrane phospholipids that may cause dysfunction of dopaminergic and serotonergic neurotransmitter receptors
(2). Preliminary reports have indicated symptom improvement ranging from 17% to 85% when omega-3 fatty acids were added to patients’ usual medications
(2–
4). In this study we evaluated the efficacy of 3 g/day of ethyl eicosapentaenoic acid (EPA) in a 16-week, double-blind, placebo-controlled trial involving schizophrenia patients with clinically significant residual symptoms.
Method
Subjects were outpatients between ages 18 and 65 who met DSM-IV criteria for schizophrenia or schizoaffective disorder. Additional inclusion criteria were the following:
1. No change of medication in the prior 30 days and no future change anticipated.
2. Pharmacological treatment that conforms to Schizophrenia Patient Outcomes Research Team
(5) recommendations 1, 2, 7–9, 12, 17, and 18 (from the U.S. Agency for Health Care Policy and Research).
3. Presence of significant residual symptoms (defined as either one or more positive and/or negative symptom scores greater than 4 or total scores greater than 45 with a score of three or greater on at least three positive or negative items on the Positive and Negative Syndrome Scale
(6). Patients were excluded if they met criteria for substance dependence or mental retardation, had a bleeding disorder, or were taking fish oil supplements, anticoagulants, cholestyramine, or clofibrate antilipemic agents.
After complete description of the study to the subjects, written informed consent was obtained. The patients were assessed with the Positive and Negative Syndrome Scale
(6), the Abnormal Involuntary Movement Scale (AIMS)
(7), the Simpson-Angus Rating Scale
(8), the Montgomery-Åsberg Depression Rating Scale
(9), and the Clinical Global Impression scale (CGI)
(10). Assessments were repeated with raters blind to patient treatment group at weeks 1, 2, 4, 8, 12, and 16. The Repeatable Battery for the Assessment of Neuropsychological Status
(11) was administered at baseline and at week 16. Reports of adverse events were elicited with open-ended questioning at each study visit. The Willet Dietary Survey
(12) was used to estimate baseline fatty acid consumption, and fasting RBC fatty acid composition was quantified by use of capillary gas chromatography with flame ionization detection (HP6890, Hewlett-Packard, Wilmington, Del.) after fatty acid extraction from washed RBC. The ratio of arachidonic acid (AA) to eicosapentaenoic acid (AA/EPA) in RBC membrane was used to index pre- to posttreatment fatty acid composition of RBC membrane.
After baseline assessment, the patients were randomly assigned to receive six identical capsules per day of vitamin E, 4 mg (to retard spoilage), and either 500 mg of ethyl EPA or a mineral oil placebo supplied by Laxdale, Ltd. (Stirling, U.K.). Three grams of EPA was chosen as a dose within the range that has been reported effective in prior studies and is generally recognized as safe
(2). The patients were instructed to take six capsules daily with meals. Adherence was monitored by pill count and RBC fatty acid quantification. The patients were unable to distinguish the tasteless and odorless ethyl EPA from placebo.
Patients randomly assigned to EPA treatment and placebo were compared across group variables by using two-tailed t tests or chi-square analyses. An alpha level of 0.05 was considered statistically significant for these comparisons.
The patients who completed the week 1 assessment were included in our evaluation of efficacy. Primary outcome analyses consisted of repeated measures analyses of variance (ANOVAs) for total scores on the Positive and Negative Syndrome Scale, the CGI scale, the Montgomery-Åsberg Depression Rating Scale, the AIMS, the Simpson-Angus Rating Scale, and the Repeatable Battery for the Assessment of Neuropsychological Status. Secondary analyses included comparisons of psychopathology subscale scores on the Positive and Negative Syndrome Scale and simple correlations between changes in efficacy ratings and between changes in AA/EPA ratios from baseline to week 16. Data were analyzed for the intention-to-treat group with use of the last observation carried forward and were repeated for the subgroup meeting a priori criteria for trial completion, who 1) had no increase in neuroleptic medication administered over the 16-week trial and 2) had received at least 12 weeks of double-blind treatment.
Results
Ninety patients entered the study; three withdrew their consent in the first week of the study (two receiving EPA and one receiving placebo), resulting in 87 patients in the last-observation-carried-forward group. Eight patients required an increase in neuroleptic dose over the trial (four receiving EPA and four receiving placebo), and four were terminated from the study before week 12 (two receiving drug and two receiving placebo) for nonadherence to study medication guidelines. Thus, 75 patients (37 receiving EPA and 38 receiving placebo) met the criteria for trial completion.
The patients’ average age was 40 years (SD=10); 61% (53 of 87) were male, 80% (N=70) were single, and 84% (N=73) were Caucasian. Most of the patients (N=79, 91%) were high school graduates. The patients met criteria for schizophrenia (N=61, 70%) or schizoaffective disorder (N=26, 30%), first became ill at the mean age of 20.8 years (SD=6.3), were first hospitalized at a mean age of 21.8 years (SD=7.6), and had a mean of 10.7 prior hospitalizations (SD=4.1). All but one patient were prescribed a neuroleptic; 19 (22%) were taking two neuroleptics, 34 (39%) were taking risperidone, olanzapine, or quetiapine, and 24 (28%) were taking clozapine.
Trial completers (N=75) and noncompleters (N=12), as well as groups receiving EPA (N=43) and placebo (N=44), did not differ significantly on any patient characteristics, including consumption of omega-3 fatty acid in their daily diet (mean=367 mg/day, SD=378). Mean baseline-to-week 16 changes in AA/EPA ratios of the RBC membranes in patients treated with EPA and in patients given placebo were –29.4 (SD=11.3) and–4.0 (SD=12.2), respectively (t=–9.21, df=71, p<0.001).
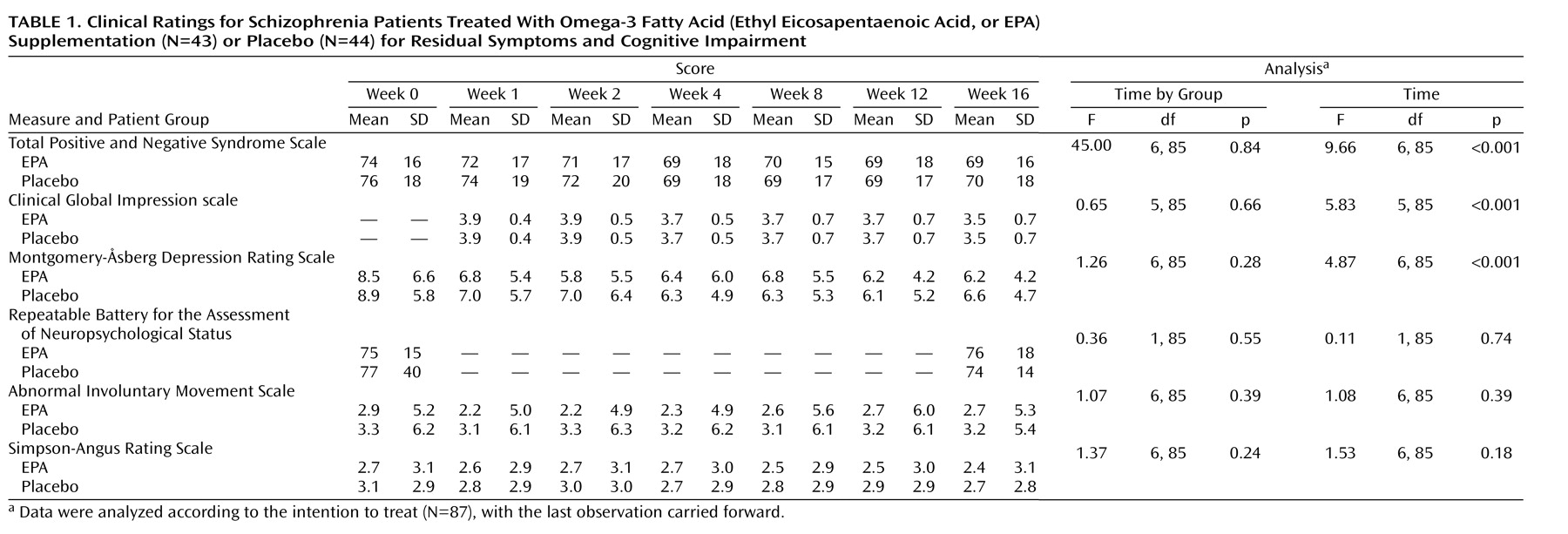
Repeated measures ANOVA indicated a small but significant effect for time for patients treated with EPA and patients given placebo on total scores on the Positive and Negative Syndrome Scale, the Montgomery-Åsberg Depression Rating Scale, and the CGI scale, but we found no time-by-group interaction effect (
Table 1). Neither interaction effects for time nor for time-by-group were observed for ratings of cognition, extrapyramidal symptoms, and tardive dyskinesia. No differences were found in subscale scores on the Positive and Negative Syndrome Scale, and the results were found to be unchanged among the trial completers. The AA/EPA ratio change from baseline to week 16 was not significantly associated with any efficacy variable. Two adverse events (upper respiratory infection [8 of 43, 19%] and diarrhea [8 of 43, 19%]) occurred among more than 5% of EPA-treated patients.
Discussion
Lipid membrane hypotheses of schizophrenia have heuristic appeal, and preliminary reports of therapeutic efficacy for omega-3 fatty acids are intriguing
(2). Unfortunately, we found no evidence for efficacy of supplemental EPA beyond that of placebo for symptoms, cognitive impairment, mood, or extrapyramidal syndromes of schizophrenia. The placebo response reported here is of sufficient magnitude to explain the effect of EPA in open trials.
Although this is a negative study, it was carefully conducted and adequately powered to detect a moderate effect size. Our patients, however, had been ill for two decades and still had substantial symptoms, despite treatment with newer neuroleptics, including clozapine. The patients described as benefiting from EPA in prior studies were younger and had a shorter duration of illness. Thus, testing of omega-3 fatty acid supplementation among less severely ill patients earlier in their illnesses may be warranted. It is also possible that a different EPA dose or treatment duration might yield different results.
Because schizophrenia is a serious disorder for which available treatments are often inadequate, reports of efficacy of newer therapeutic compounds generate considerable interest. In open trials this enthusiasm may facilitate placebo response. Newer therapies for treatment-resistant conditions should be rapidly evaluated in double-blind, placebo-controlled trials.