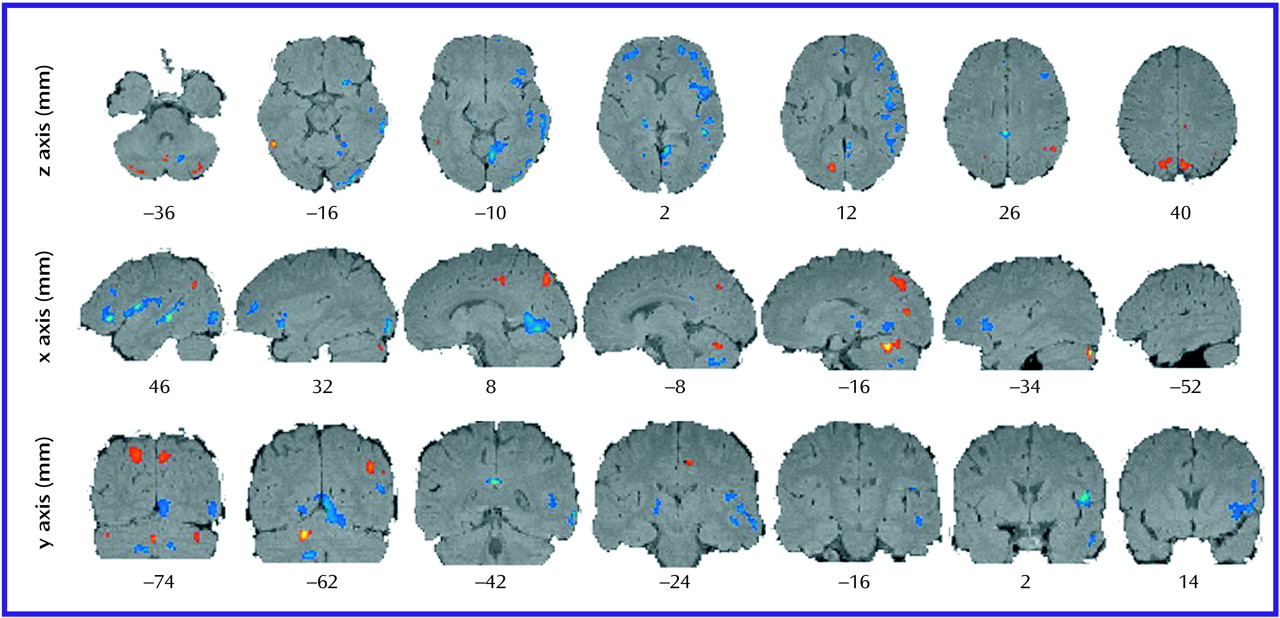
Methodological Considerations
The lack of precise anatomical localization with PET is a recognized problem. Anatomical localization can be addressed by using atlas brain coordinates or by using individual MRI templates coregistered with individual PET scans. However, individual MRI templates were not available for this study. We used statistical parametric mapping analysis with Talairach and Tournoux coordinates
(20) for localization, but this method does not consider continuity of structure and shape analysis in identifying anatomical coordinates and associated structural nomenclature. Thus, an additional independent analysis of anatomical localization was done by a neuroanatomist (J.H.F.) who was blind to the subjects’ group assignments. The neuroanatomist used known locations and considered three-dimensional shapes spanning adjacent slices.
Anatomical localizations resulting from both the statistical parametric mapping analysis and the neuroanatomist’s analysis are presented in
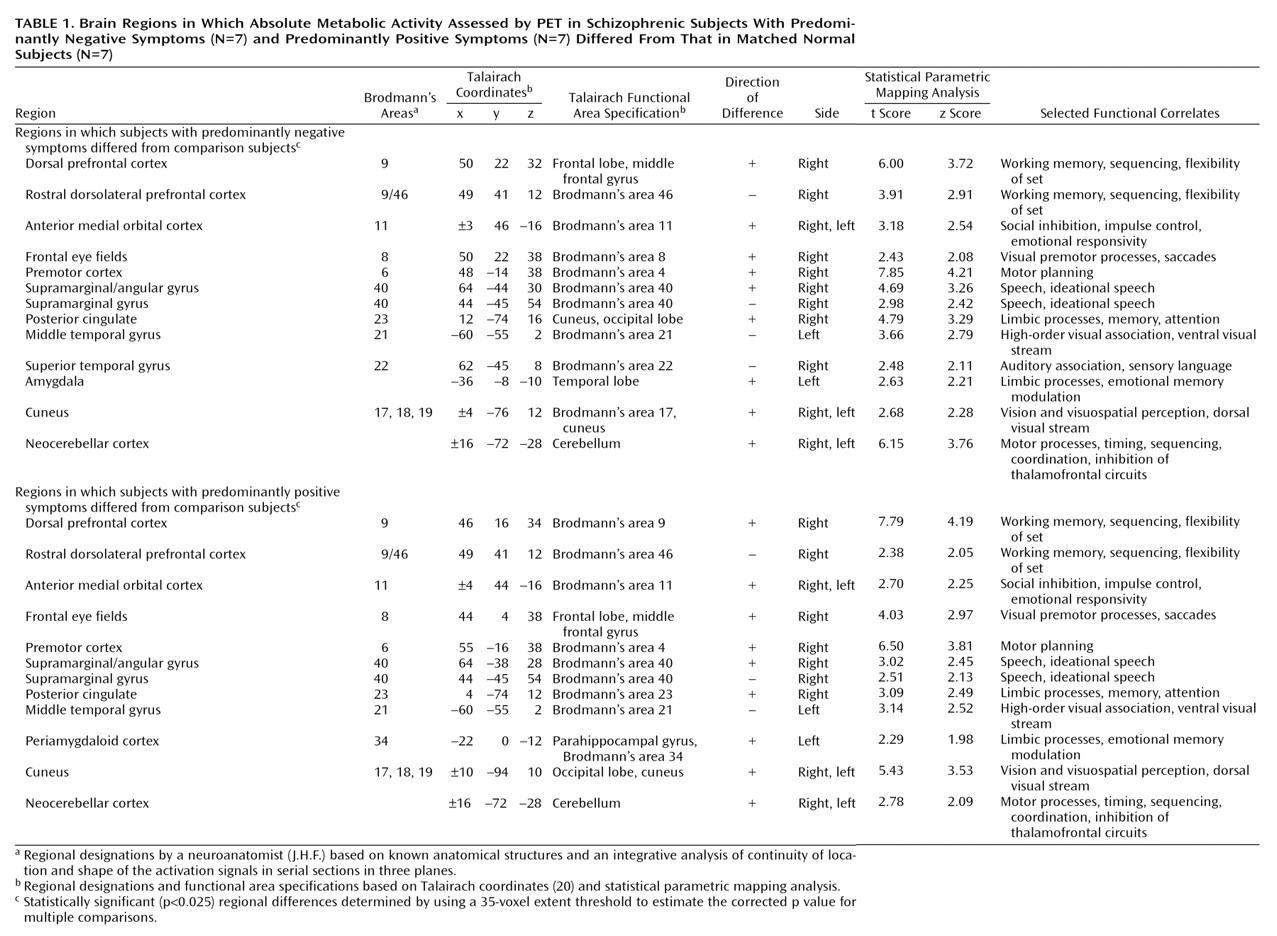
Table 1 and
Table 2. Eighty percent of the localizations developed by the two methods were equivalent. Five percent were not equivalent because of different designations of the border between the cerebellar cortex and the inferomedial occipitotemporal cortex. In these cases, the neuroanatomist considered the continuity of the activation signals across three to five sections that revealed “migration” of signals in two directions—one toward an unequivocal cerebellum and the other toward an unequivocal occipitotemporal cortex. Thus, in the statistical parametric mapping analysis that used Talairach coordinates, localization of the signal at the single local maximum within a cluster underestimated the true extent of the entire signal. In the other cases of disagreement (mostly in the temporal lobe), the maximum resulting from the statistical parametric mapping analysis with Talairach coordinates was located at the border of two cortical areas, e.g., at the depth of a sulcus or at the border of white matter and an identified structure. Again, the neuroanatomist followed the activation signal through several adjacent sections and compared the result to the maximum inside the cluster derived from the statistical parametric mapping analysis. The single local maximum was within the anatomical area but did not represent the entire activation cluster as one moved from anatomical section to section.
Partial volume effects, due to enlarged ventricles or cortical thinning, might explain the lower levels of glucose metabolism in some regions. The areas of difference between the subjects with predominantly negative symptoms and those with predominantly positive symptoms, however, are not periventricular and therefore are less likely to be subject to partial volume effects related to ventricular enlargement. In addition, the areas with structural differences reported in schizophrenia (including in the superior temporal gyrus, medial temporal lobe, thalamus, basal ganglia, corpus callosum, and vermis of the cerebellum [
24–
26]) were not the areas that uniquely distinguished the negative symptom subjects from the positive symptom subjects in this study. The cerebellar/inferior occipital lobe border was the one area where unequivocal assignment of location was most problematic. No established structural changes in the inferior occipital lobe, the neocerebellar cortex, or deep cerebellar nuclei suggested partial volume confounds. The structural findings associated with the cerebellum have been confined to the vermis
(26). The lack of signal localization in the ventricle or external to the brain substance also argued against partial volume effects underlying our findings.
Our PET study groups were relatively small; however, the subjects were matched between the three groups for gender, age, and handedness. The small size of the study groups may have explained why we did not find statistically significant differences between groups in performance on the Continuous Performance Task. In addition, it is often difficult to separate depression from negative symptoms and from the deficit syndrome
(7). We excluded subjects who had evidence of depression from the negative symptom group. However, we did not assess whether subjects met the criteria for the deficit syndrome
(7). Our findings may not extend to schizophrenic patients with deficit syndrome, concurrent depression, or a treatment-refractory illness. Further, we only studied right-handed male schizophrenic subjects. Therefore, we have not explored potential gender or handedness differences.
The FDG PET activation task used in this study, a degraded Continuous Performance Task, requires the subject to exercise visual perception, discrimination, perception of salience, attention, and cognition. No statistically significant differences in performance on the Continuous Performance Task were found between the positive and negative symptom groups, but, given the small size of the groups, some of the observed differences in glucose metabolic rate could reflect differences in Continuous Performance Task performance. However, this possibility was accounted for in the statistical parametric mapping analysis by covarying for Continuous Performance Task performance. Thus, differences in performance are unlikely to account for the observed differences in metabolic rate. Further, this study substantiates the value of a controlled cognitive task in revealing the circuitry underlying normal brain function and the pathophysiological mechanisms of neuropsychiatric disorders.
Significance of Findings
The negative symptoms of schizophrenia are difficult to treat, and the pathophysiological mechanisms underlying negative symptoms have not been adequately described. The present study was undertaken to examine the pattern of differences in glucose metabolic rate in subjects with predominantly negative symptoms, compared with subjects with predominantly positive symptoms.
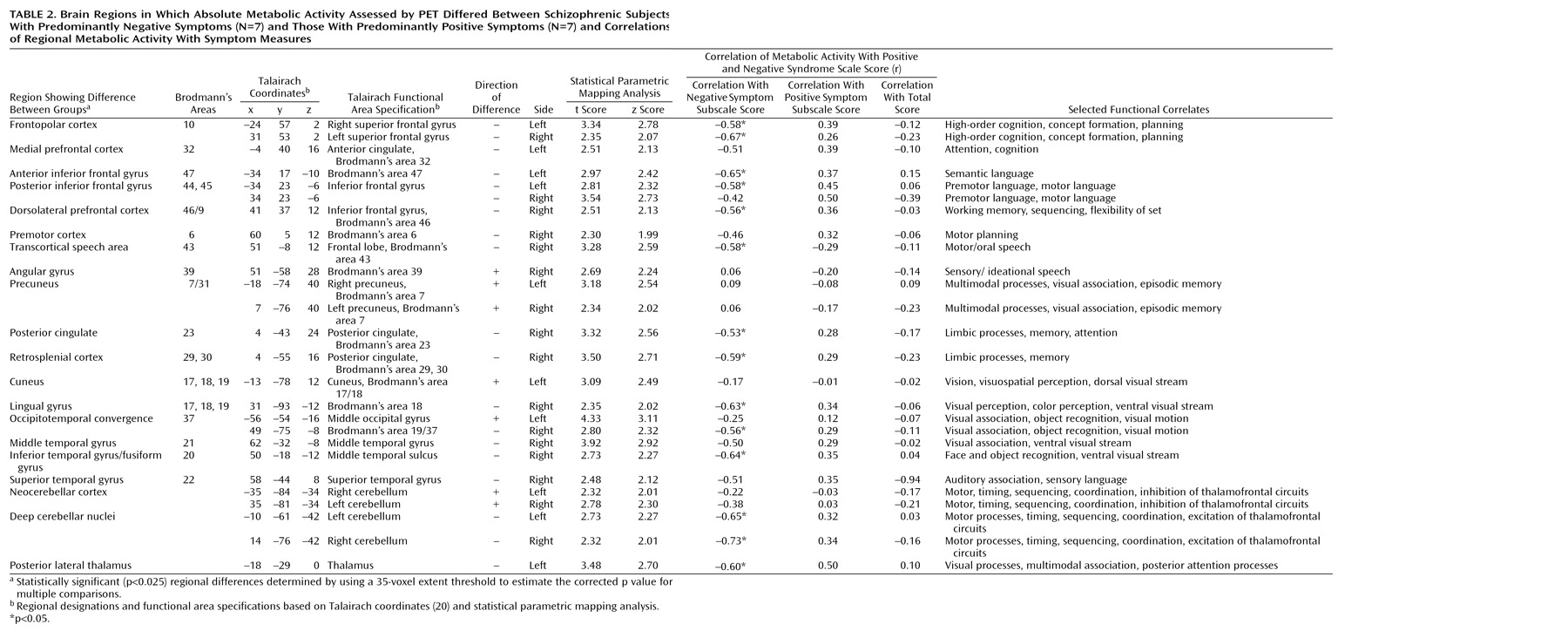
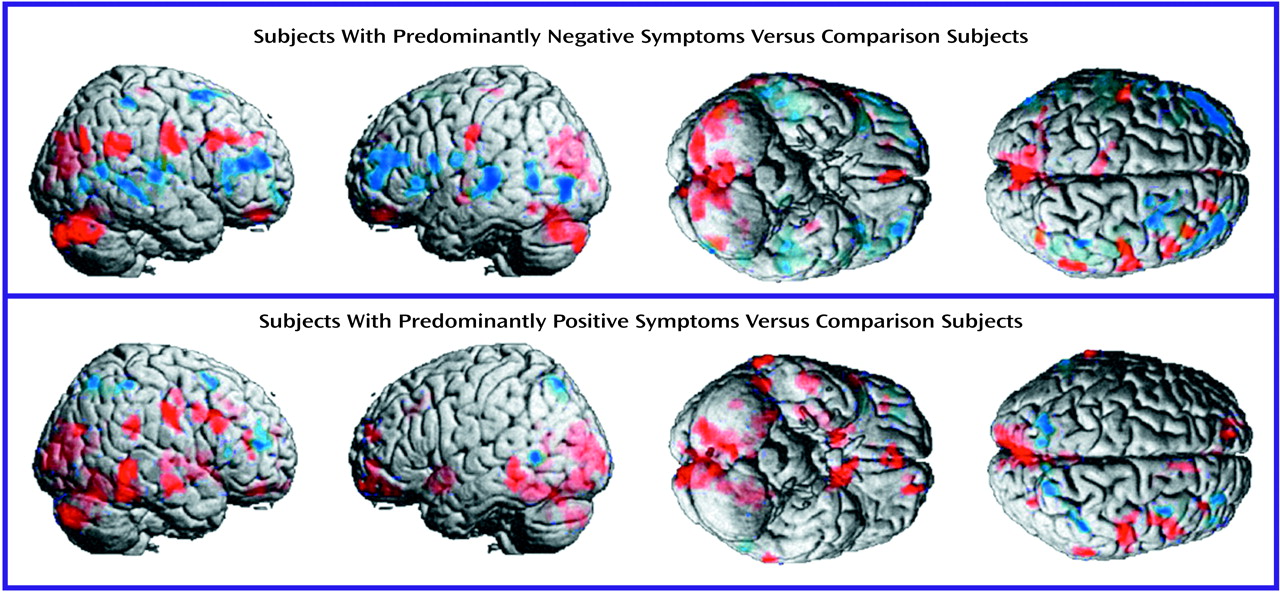
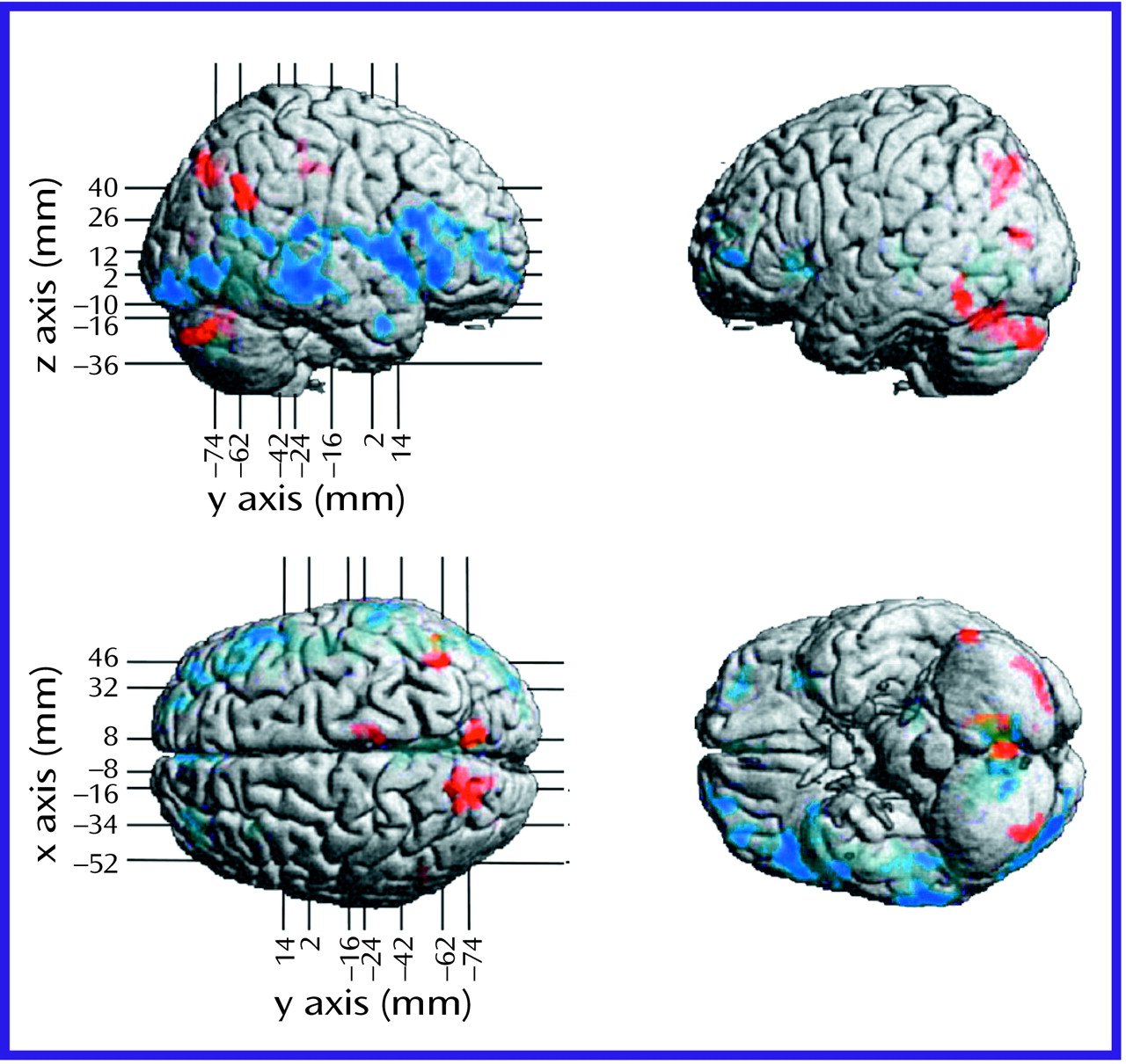
The negative symptoms of schizophrenia include attentional impairments, impoverished emotional expression, social withdrawal, and the inability to develop good rapport. Patients with predominantly negative symptoms are characterized as having blunted affect and as apathetic and passive. It is expected that these symptoms would be reflected in abnormalities in several well-described neural systems. Attentional impairments may be reflected in changes in the medial prefrontal, anterior cingulate, and dorsolateral prefrontal regions. In this study, significant differences between the negative symptom subjects and those with predominantly positive symptoms, as well as the comparison group, were indeed observed in the medial prefrontal, dorsolateral prefrontal, and posterior cingulate cortices (but not the anterior cingulate cortices). These differences were most apparent in the subjects with predominantly negative symptoms, as reflected by their lower glucose metabolic rates in these areas.
The lower levels of emotional expression in patients with negative symptoms may be reflected in the strikingly lower glucose metabolic rates in the ventral prefrontal and orbital cortices. These differences were most apparent on the right side of the brain, the side that subserves the preponderance of emotional expression.
Language is affected more severely in subjects with predominantly negative symptoms than in those with predominantly positive symptoms. Not only are the amount and content of language impoverished in patients with negative symptoms, but the emotional expression and prosody are markedly poorer, contributing to the flat affect characteristic of the negative symptom patients. These symptoms are consistent with the dramatically lower metabolic levels in the sensory associative (Brodmann’s area 22), transcortical (Brodmann’s area 43), premotor (Brodmann’s areas 45 and 44), and motor (Brodmann’s areas 4 and 6) language cortices on the right side of the brain in the negative symptom subjects in this study. One language-associated cortex that had a higher level of metabolism in the negative symptom subjects is Brodmann’s area 39 on the right side, suggesting that at least part of the speech circuitry can be activated to a greater degree in negative symptom subjects.
We observed increases in the visual eye fields (Brodmann’s area 8) and the occipitotemporal convergence zone (Brodmann’s area 21 at the border of area 37) possibly overlapping V5 visual cortical area for both subjects with predominantly negative symptoms and those with predominantly positive symptoms. Perception of motion and visuospatial function are not well studied in schizophrenic patients. Stuve and colleagues
(27) concluded that a dysfunction in V5 leading to a deficit in motion perception could explain the deficit in smooth pursuit gain observed in schizophrenia. Saccadic
(28) and exploratory eye movement deficits and visual tracking abnormalities are well documented in schizophrenia and reported to be greater in patients with negative symptoms by some investigators
(29,
30).
Patients with negative symptoms are especially subject to difficulties in correctly identifying and expressing the emotional content of both faces and scenes
(31). Consistent with this symptom was the lower glucose metabolic rate in the ventral visual stream/inferotemporal/fusiform area in the right hemisphere that we observed in the subjects with predominantly negative symptoms. By using facial emotion recognition tests, Borod and colleagues
(32) provided support that negative symptoms in schizophrenia are associated with right hemisphere dysfunction, a finding consistent with the present results.
The majority of theories about the etiology and treatment of schizophrenia implicate dopaminergic mechanisms. Subcortical hyperdopaminergic function in schizophrenia, with concomitant hypometabolism in the prefrontal cortex and striatum, has been a dominant theme, reflecting dysfunctional cortical-striatal-pallidal-thalamic-cortical loops
(33). Our data suggest an even greater metabolic deficit in the prefrontal and temporal cortices in the subjects with predominantly negative symptoms, which is consistent with a greater cortical dopamine dysfunction in these subjects. The lack of subcortical differences between the negative symptom subjects and the positive symptom subjects is noteworthy.
The negative symptom subjects had a higher glucose metabolic rate in the cerebellar cortex bilaterally but a lower rate in the deep cerebellar nuclei. These cerebellar abnormalities are consistent with a higher level of neocerebellar inhibition of deep cerebellar nuclei, leading to a lower level of excitation of cerebellar-thalamic-frontal output. Thus, the lower metabolic rates bilaterally in the frontal lobes of subjects with predominantly negative symptoms are consistent with cerebellar output dysfunction or “attempts” to compensate for the primary frontal deficit.
Cognitive dysmetria, described by Andreasen and colleagues
(34) as an impairment in synchrony of mental processes, has been ascribed to dysfunction in the cortical-thalamic-cerebellar-cortical circuit. This feedback loop permits a “constant checking and updating of input and output at the nanosecond level, and facilitates the rapid and smooth execution of complex motor acts;” it also contributes to “monitoring and coordinating the fluid execution of mental activity”
(34). In general, subjects with predominantly negative symptoms have greater deficits in these motor and cognitive operations. Although most patients have both positive and negative symptoms cross-sectionally, our study groups were chosen on the basis of a predominance of symptoms in order to facilitate contrasts in symptoms, functions, and associated circuitry. It is possible that the higher cerebellar metabolic rates in the negative symptom subjects constituted compensation to overcome a relative failure of activation of the corticocortical, corticostriatal, and associated basal ganglia loop circuitry, especially in the frontal lobe. This interpretation is consistent with the positive correlation between cerebellar blood flow and number of negative symptoms found by Kim et al.
(10). The negative symptom subjects appeared to have more abnormalities of these circuits than the positive symptom subjects. Further studies involving subjects with schizophrenia and schizoaffective disorder who manifest both prominent positive and negative symptoms are needed to validate and extend our findings.