Bipolar II disorder, recognized as a distinct type of affective illness
(1), has not been well characterized in terms of underlying brain dysfunction. Although the demonstration of resting state changes in bipolar I disorder suggests profound alterations in brain function
(2), it does not address the dynamic changes associated with cognitive tasks. Both higher measures of novelty-seeking in patients with bipolar disorder
(3) and the association of novelty-seeking with poor treatment outcome
(4) suggest a linkage to novelty in bipolar spectrum disorders, raising the possibility that patients with bipolar disorder have a hypersensitivity to novelty. Therefore, we designed a neuroimaging task that confronted subjects with novel finger movement sequences and compared the dynamic brain response in patients with bipolar II disorder with that of healthy comparison subjects. The task had the advantage of being affectively neutral because the stimuli themselves had no intrinsic emotional valence, isolating the effect of novelty.
The serial reaction time task taps visuospatial attention and motor learning and has been extensively studied with neuroimaging
(5). Subjects are repeatedly cued to one of several possible button presses, but the order of button presses follows a sequence. Subjects get faster as aspects of the sequence are encoded. When the sequence is changed, both attentional and subcortical regions become transiently activated.
Method
Thirteen right-handed outpatients who met DSM-IV criteria for bipolar II disorder and 14 healthy comparison subjects were recruited from two institutions: the University of Pittsburgh and Emory University. The mean age of the patients was 31.2 years (SD=8.3, range=21–47); the mean age of the comparison subjects was 30.8 (SD=8.3, range=20–43). The male-female ratio among the patients was 5:8; among the comparison subjects it was 6:8. Patients were euthymic at the time of study and had no history of substance abuse within the last year. Antidepressants and mood stabilizers were allowed, but no patients were taking antipsychotics. Four of the patients were medication free, two were taking lithium plus sertraline, two were taking divalproex, and one each was taking lithium, divalproex plus sertraline, divalproex plus citalopram, paroxetine, and lamotrigine plus bupropion. After complete description of the study, all subjects gave written informed consent. This study was approved by the institutional review boards and radiation committees of both the University of Pittsburgh and Emory University.
Fourteen measurements of regional cerebral blood flow (rCBF) were obtained by using positron emission tomography (PET) following intravenous bolus injection of [15O]H2O. Imaging was performed on a Siemens HR+ (New York) at Pittsburgh (one patient, six comparison subjects) and a Siemens ECAT 921 at Emory (12 patients, eight comparison subjects). The dose of [15O]H2O was 12 mCi on the HR+ and 25 mCi on the 921. Imaging was performed in three-dimensional mode on both scanners with measured attenuation correction on the HR+ and calculated correction on the 921. Following injection of [15O]H2O, each scan lasted 90 seconds; the interscan duration was 8–10 minutes.
Subjects were instructed to perform a reaction time task while in the scanner. A computer displayed four boxes arrayed horizontally from left to right. Every 1400 msec one box was illuminated, and the subject had to press a corresponding key on the keyboard as quickly and accurately as possible. The order of the illuminated boxes followed a 12-item sequence that repeated seven times during the course of a 2-minute trial block. A 2-minute rest period between blocks was provided, during which subjects received feedback about their performance. Twenty-seven blocks were performed, and scans were obtained on the odd-numbered blocks. One sequence (sequence A) was used throughout a given subject’s task except during blocks 13, 19, 21, and 27, in which a different 12-item sequence was presented (sequence B). This allocated the first half of the task for the acquisition of sequence A, and the introduction of sequence B in the latter half allowed the measurement of rCBF changes in response to a novel spatial-motor mapping.
PET images were analyzed to determine changes in rCBF associated with the change in sequence and whether this was different for patients and comparison subjects. Using statistical parametric mapping (SPM 99 [Wellcome Department of Cognitive Neurology, University College London]), we corrected images for interscan head movement, spatially transformed to the Montreal Neurological Institute coordinate system and spatially smoothed with a 12-mm Gaussian filter. A single contrast image for each subject was formed by the difference between the mean adjusted scans of sequence B (blocks 13 and 19) and sequence A (blocks 9, 11, 15, 17, 23, and 25). This difference represented the change in rCBF associated with the novel sequence after the subject had acquired the first sequence. To capture the maximal effect of novelty, only the first two blocks of sequence B were used to calculate the change in rCBF. The single contrast image for each subject was entered into a random effects analysis with a main effect of group. We then examined the interaction of group-by-sequence change, with a threshold for statistical significance of p<0.001 (uncorrected for multiple comparisons).
Results
Complete reaction time data were collected for 23 subjects (10 patients and 13 comparison subjects). Both patients and comparison subjects displayed an increase in mean reaction time when the sequence was changed (mean increase=29.2 msec, SD=4.9) (F=35.0, df=1, 21, p<0.001, repeated measures analysis of variance), but this increase was not significantly different between groups (F=1.98, df=1, 21, p=0.17). The mean reaction time was not significantly different between the groups (F=1.33, df=1, 21, p=0.26).
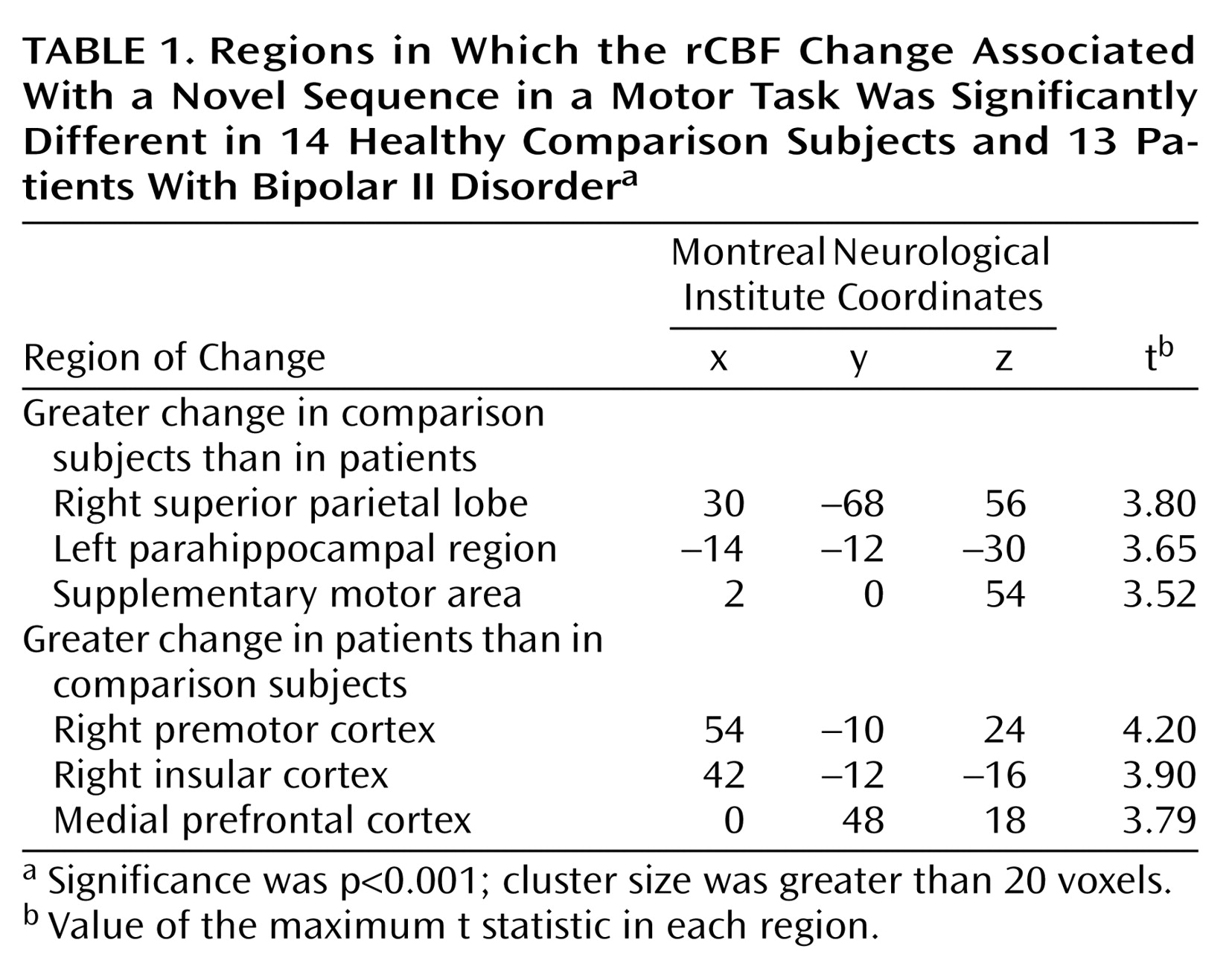
In the rCBF data, the group-by-sequence change interaction revealed several areas that differed between the groups (
Table 1). Comparison subjects displayed a significantly greater increase than the patients in the right superior parietal cortex when the sequence was changed. In contrast, the patients displayed a greater increase than the comparison group in a widespread network that included the right premotor cortex, right insular cortex, and medial prefrontal cortex.
Because imaging data were collected on two different scanners, we tested for the possibility of the scanner as a confound. There was no significant difference in the group distribution between the two scanners (Yates-corrected χ2=2.71, df=1, p=0.10), nor were there any areas in the rCBF analysis that had significant scanner-by-sequence change interactions at p<0.001.
Discussion
Both patients and comparison subjects were able to encode aspects of the motor sequence, but when the sequence was changed they showed different brain responses. This difference in brain activation occurred despite the fact that both groups had comparable behavioral responses. Because of the confound of medications in the patient group, it is possible that all of the observed differences were attributable to medication, but the comparable behavioral data show that the medications were not enough to impair performance.
The activation of the right superior parietal cortex in response to a novel spatial-motor sequence is consistent with a large body of literature supporting the role of this region in spatial attention
(6). The superior parietal activation was likely related to a shift in spatial attention, but this was significantly more active in the comparison subjects. Most subjects became aware of parts of the sequence and were aware of when it changed, but this was not demonstrably different between the groups. We used a relatively simple repeating sequence, and so the development of explicit knowledge was not surprising. This may explain the lack of activation in the basal ganglia, which often occurs with more complex, implicitly learned sequences. It is possible that the degree of explicit knowledge differed between the groups, accounting for some activation differences, but differentiating knowledge from recall is notoriously difficult.
The lack of parietal activation in the patients, coupled with widespread medial prefrontal and limbic activation, suggests that the adaptation to the novel sequence occurred by a different mechanism in the patients. The task was designed to be affectively neutral, yet activation of limbic regions may be associated with an arousal function rather than an attention function. Although this does not demonstrate a hypersensitivity to novelty, it does show that patients with bipolar II disorder react biologically in a way that is congruent with the symptom of affective lability.