There is little debate as to the power of the placebo effect in controlled short-term clinical trials of antidepressants, as well as in other medical and surgical treatments
(1–
3). Placebo response in the acute phase of antidepressant trials has often been seen as an unavoidable and distracting consequence inherent in the assessment of any given treatment intervention—whether cognitive, pharmacological, or surgical
(4–
11). While continuation studies
(12–
16) have repeatedly demonstrated an advantage of maintenance medication over continued placebo administration in preventing relapse and recurrence, the presence of a significant placebo effect with short-term administration provides a unique opportunity to examine brain mechanisms mediating clinical antidepressant response unencumbered by nonspecific drug, lesion, or learning effects evoked by medication, surgery, or cognitive therapy.
Positron emission tomography (PET) measures of regional glucose metabolism have proven to be sensitive indices of brain function in patients both in the untreated depressed state
(17–
24) and after disparate treatments
(24–
38). Functional changes in cortical (dorsal and ventral prefrontal, anterior temporal, inferior parietal), limbic-paralimbic (anterior, subgenual, and posterior cingulate; hippocampus; amygdala; anterior and posterior insula) and subcortical (basal ganglia, thalamus, brainstem) regions have also been described after various types of treatments, including medication, sleep deprivation, ECT, repetitive transcranial magnetic stimulation, and ablative surgery. Normalization of frontal hypometabolism is the best-replicated finding, seen mainly with medication treatment
(25–
31). Decreases in glucose metabolism in limbic and paralimbic regions, varying with drug treatment, are more common in studies of sleep deprivation, ECT, and surgery
(29–
38). There are surprisingly few data available on functional brain changes associated with cognitive interventions
(39,
40), despite the repeated evidence that these strategies are of equal efficacy to drugs in alleviating core features in depressed patients with mild to moderately severe illness
(41–
44). Studies characterizing consistent changes common to treatment with different modes of action
(39,
40,
45) are also sparse, as is the literature concerning the variability of regional effects associated with response to different treatments
(46). We know of no PET studies of brain changes associated with placebo administration.
This study examined changes in regional brain glucose metabolism associated with placebo response in patients with major depression who were participating in a double-blind, placebo-controlled PET imaging study of the effects of the antidepressant fluoxetine
(28,
29). We hypothesized the existence of a common pattern of regional metabolic changes with clinical response, independent of whether a patient was given active or inactive medication, with response to active fluoxetine showing additional regional changes reflective of drug-specific effects.
Method
Treatment Protocol
Seventeen unmedicated depressed men (age: mean=49 years, SD=9; current episode duration: mean=18 weeks, SD=2; score on 17-item Hamilton Depression Rating Scale: mean=22, SD=5, score on Mini-Mental State Examination: mean=29, SD=2) with symptoms requiring treatment were recruited from the inpatient psychiatry unit at the Audie L. Murphy Memorial Veterans Administration Hospital in San Antonio, Tex. The clinical diagnosis of a major depressive episode, unipolar type, was confirmed by two independent psychiatrists using DSM-IV criteria and a structured psychiatric interview
(47). None of the enrolled subjects was considered treatment resistant by history. Exclusion criteria included a history of neurological disease, head trauma, or other axis I psychiatric diagnoses, as well as having current psychotic symptoms, substance abuse, antidepressant treatment within the preceding month, previous nonresponse to fluoxetine, or previous ECT. Written informed consent was obtained from all subjects, and the study was conducted as approved by the institutional review board of the University of Texas Health Science Center.
The patients were treated on an inpatient research unit for 6 weeks. The patients were randomly assigned, with use of a double-blind study design, to either fluoxetine, 20 mg/day, fixed dose, or placebo. Additionally, all patients received the therapeutic benefits of the standard ward milieu, which included daily individual meetings with the treating physician, group therapy, and various ward activities. The patients did not receive interpersonal psychotherapy or cognitive behavior therapy during the 6 weeks. The patients, treating physicians, ward personnel, and PET imaging team remained blind to the specific substance received during the full 6-week study.
Imaging Studies
Regional cerebral glucose metabolism was measured with standard methods
(48) in all patients by using [
18F]fluorodeoxyglucose (FDG) and PET before and after 1 and 6 weeks of administration of fluoxetine or placebo. For each scan, a 5-mCi dose of FDG was injected intravenously, with image acquisition beginning after 40 minutes (scan duration: 20 minutes; GE/Scanditronix 4096 [General Electric, Milwaukee]; 15 parallel slices; 6.5-mm, center-to-center interslice distance; measured attenuation correction with transmission scans; reconstructed with a Hann filter; final in-plane resolution: 7.0 mm, full width at half maximum). The absolute glucose utilization rate was not calculated.
All scans were acquired with the patients in the supine position, awake, in the resting state, with eyes closed and ears uncovered. The patients were checked every 10 minutes to ensure they were not asleep. The subjects were not explicitly instructed to monitor their mood state or to perform any specific cognitive task. This approach was aimed at examining regional effects associated with illness remission without the potential interpretive confounds introduced by explicit manipulation of affective or cognitive states
(29,
49). A debriefing session after the uptake period documented compliance with the instructions in all subjects. The patients did not smoke in the 60 minutes before FDG injection. An anatomical magnetic resonance imaging scan was also acquired in each subject for spatial transformation of the PET data, region-of-interest analysis, and parametric image display (Elscint Gyrex 2T-DLX [Haifa, Israel]; three-dimensional gradient/recall acquisition in the steady state; TR=33 msec; TE=12 msec; flip angle=60°; volume=256×256×127; spatial resolution=1 mm
3).
Data Analysis
Overall clinical improvement was quantified at the 1-week and 6-week PET sessions by using the Hamilton Depression Rating Scale
(50) and a computerized battery of standardized behavioral tests targeting mood, motor performance, and cognition, with special emphasis on motor performance, information-processing speed, and executive functioning
(51). Included were tests of simple and choice reaction times, versions of the Stroop and Trails tests, tests of verbal fluency, and tests of fine and gross motor coordination. Responders were defined as having a minimum of 50% decrease in Hamilton depression scale scores relative to those at baseline
(50,
52).
State-related changes in regional glucose metabolism (after treatment versus baseline) were detected by using change distribution analysis
(53) and interpreted by using coordinates and Brodmann’s areas from the Talairach and Tournoux atlas of the brain
(54). Value and spatially normalized images were first trilinearly interpolated, resampled (60 slices, 8-mm
3 voxels), and Gaussian-filtered to a final resolution of 9.9 mm (full width at half maximum)
(55). A voxel-by-voxel subtraction using the pairs for the targeted condition was next performed for each individual. Within-subject differences were then averaged across subjects, creating a grand-mean-difference image for each contrast. A beta-2 statistic measuring kurtosis of the histogram of the difference image (change distribution curve) was used as an omnibus test to assess overall significance
(56). The beta-2 test was implemented with MIPS software (Research Imaging Center, San Antonio, Tex.) in a manner similar to that of the gamma-2 statistic
(53). The beta-2 improves on the gamma-2 by using a better estimate of the degrees of freedom, i.e., the number of “resyls” in the PET images
(57). The omnibus test was followed by a maxima and minima search to identify local extrema within a search volume measuring 125 mm
3 (58).
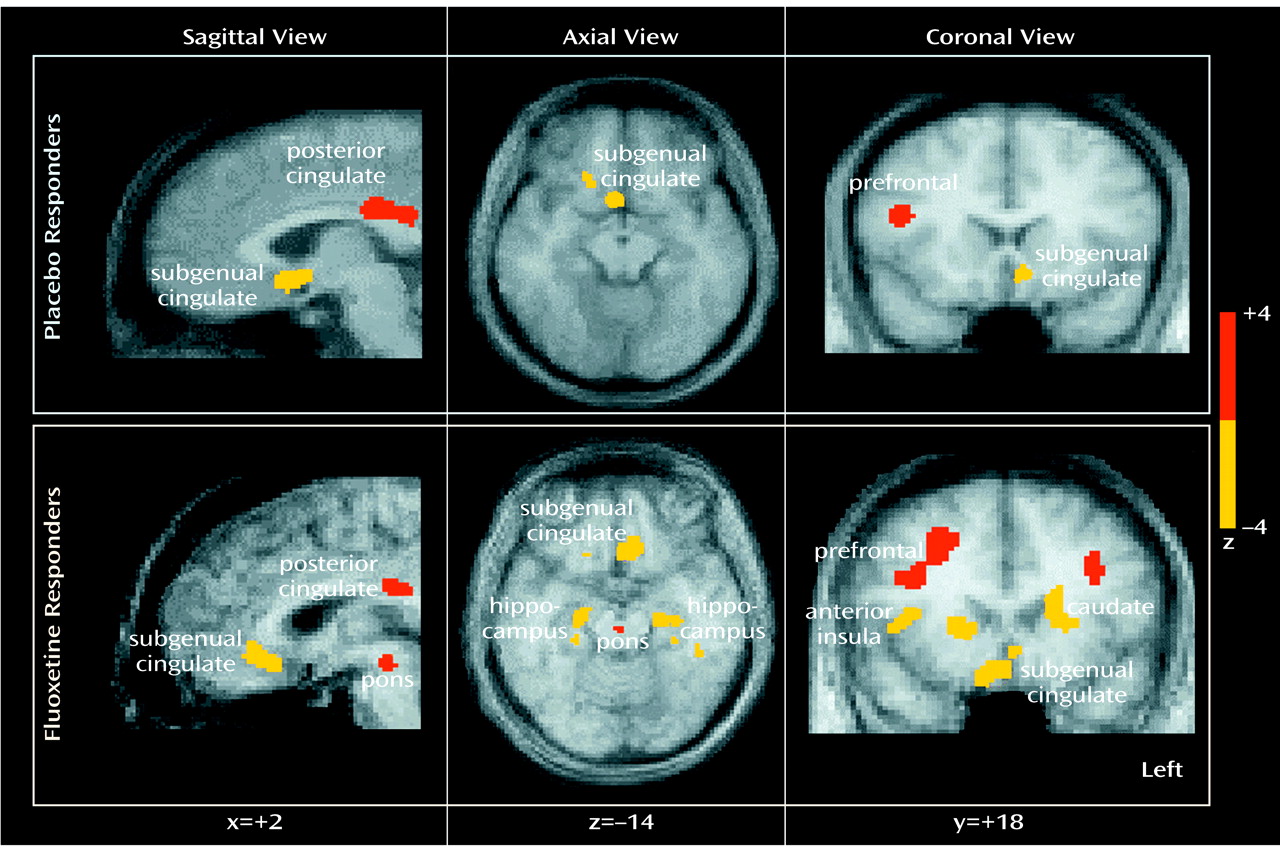
To facilitate reporting and visualization of these maxima and minima, group-mean-subtraction images were converted post hoc to statistical parametric images of z scores on the basis of the variance of all local changes within the subtraction images (
Figure 1). Locations of focal maxima and minima exceeding a z score of 2.6 (p<0.01) were determined, with the peak voxel of each area described in x, y, and z coordinates as millimeters relative to the anterior commissure.
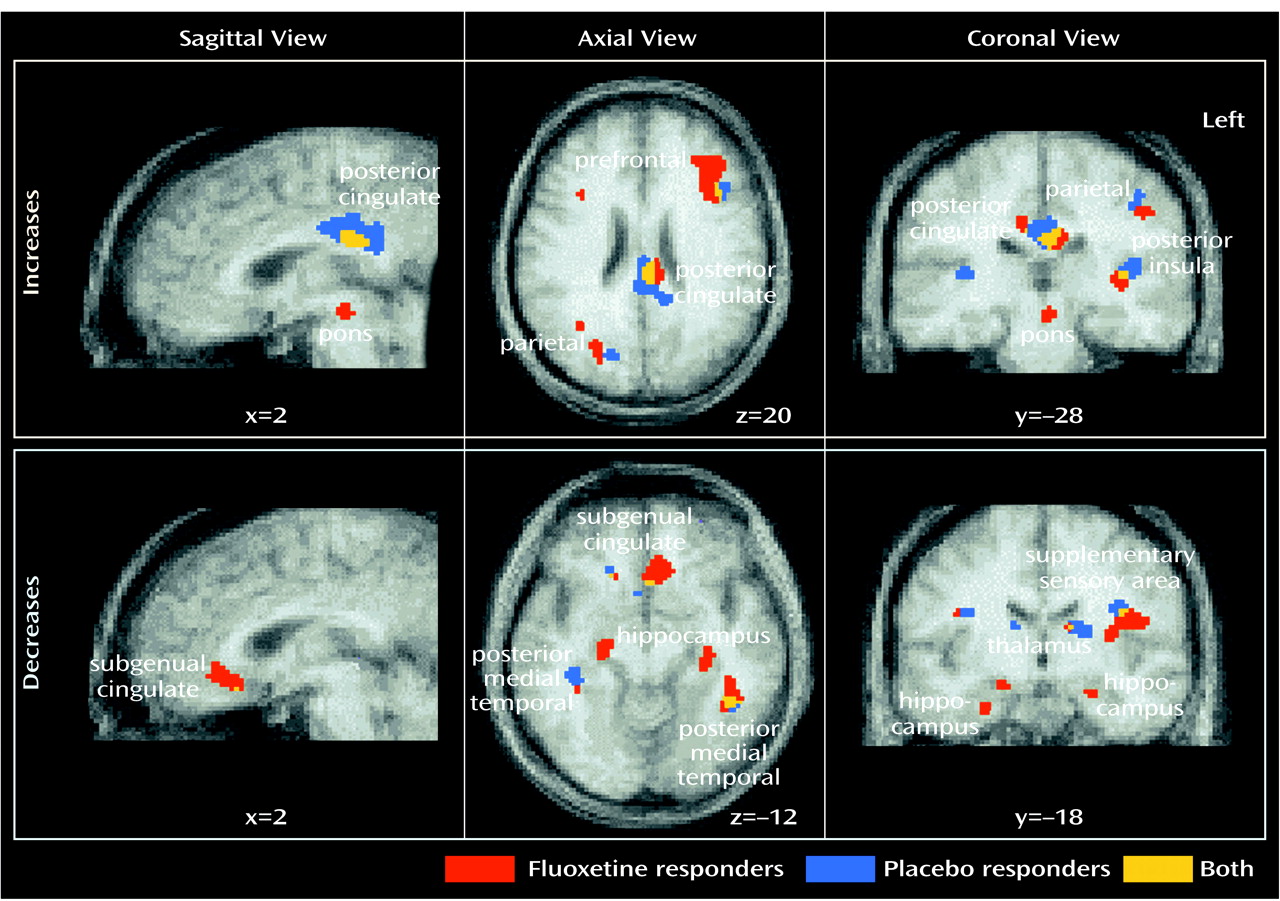
Six-week versus baseline and 1-week versus baseline comparisons were computed separately for placebo-responder and drug-responder groups. The 6-week response effect with placebo administration was the primary focus of this study. Anatomical concordance (and discordance) of focal maxima and minima across contrasts (response effect: changes in placebo versus fluoxetine responders) was defined as common (or different) Brodmann’s area identifiers for the coordinate locations
(59), as well as evidence of anatomical overlap in the extent of significant activations or deactivations seen with the use of a logical contrast of two z score maps (
Figure 2)
(28). Comparisons of 1-week versus 6-week regional metabolic changes in fluoxetine-treated patients (both responders and nonresponders) were the subject of a previous report
(29).
Results
Symptom remission was seen in eight of the 15 study completers. Breaking of the blind revealed that four of the eight responders had been treated with placebo and four with active drug. Mechanical problems with the PET camera precluded the timely scanning of two of the original 17 subjects, both of whom remained clinically symptomatic at 6 weeks and later proved to have been given placebo.
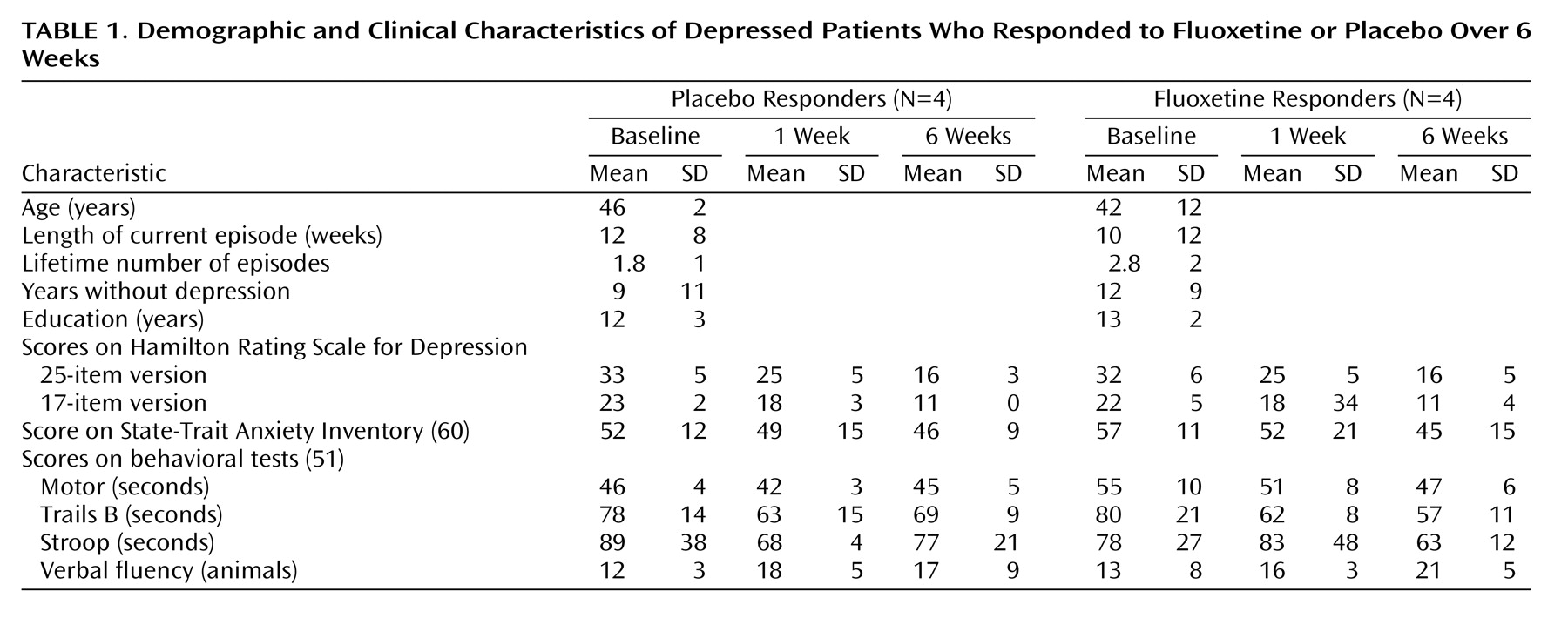
Clinical improvement was comparable for the placebo- and fluoxetine-responder groups, and there were no differences in pretrial demographic or illness characteristics or in cognitive performance variables (
Table 1). There were no significant differences after 1 week of the trial on any clinical variable that predicted 6-week clinical outcome in either group or distinguished patients given placebo from patients given drug. Factor subscores on the Hamilton depression rating scale
(61) evaluating mood, sleep, and somatic symptoms also did not distinguish the two groups.
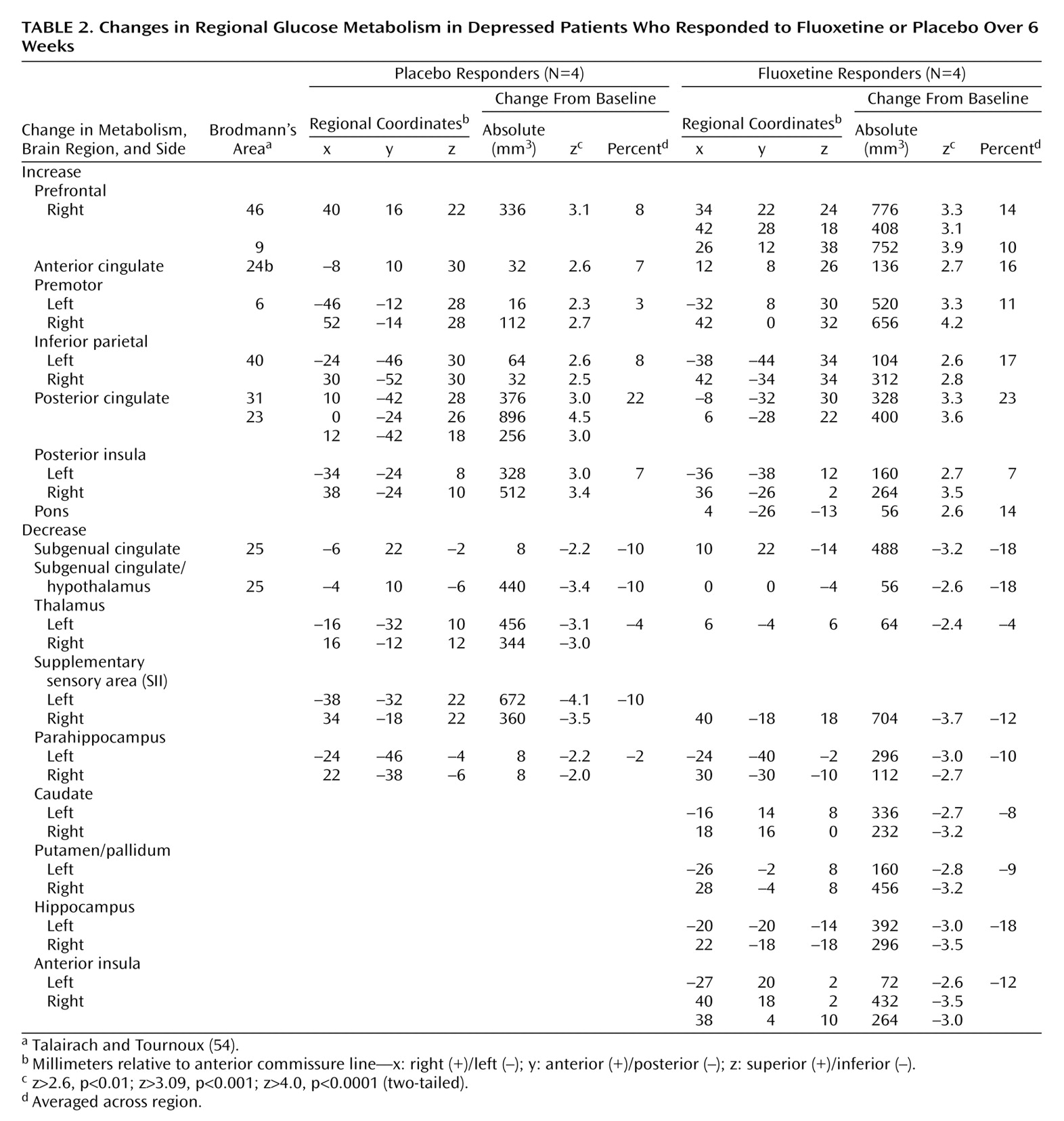
Placebo response at 6 weeks was associated with significant regional metabolic changes (scan 3 versus baseline metabolism: beta-2=3.97, df=1972, p<0.0001). Both increases and decreases in glucose metabolism that involved both neocortical and limbic-paralimbic regions were identified (
Figure 1, top). Areas of significant increases in metabolism were seen in the prefrontal cortex (Brodmann’s area 9/46), premotor cortex (Brodmann’s area 6), inferior parietal cortex (Brodmann’s area 40), posterior insula, and posterior cingulate (Brodmann’s area 23/31). Decreases in metabolism were localized to the subgenual cingulate (Brodmann’s area 25), hypothalamus, thalamus, supplementary sensory area insula, and parahippocampus (
Table 2, left).
One-week metabolic changes in placebo responders did not meet the omnibus threshold for significance. Placebo nonresponders were not evaluated because of an inadequate group size (N=3; no 6-week scan for two of the three nonresponders).
Response to active fluoxetine treatment was also associated with significant regional metabolic changes (scan 3 minus baseline metabolism: beta-2=3.44, df=1972, p<0.0006). The pattern of metabolic change closely matched that seen with response to placebo (
Table 2, right). Drug responders, however, showed additional changes in metabolism in subcortical and limbic regions (
Figure 1, bottom). Specifically, fluoxetine response was associated with unique increases in brainstem metabolism and metabolic decreases in the striatum, hippocampus, and anterior insula. There were no regional changes that were unique to placebo responders at 6 weeks.
While the locations of regional changes showed remarkable concordance across groups, the magnitude of changes with fluoxetine treatment was generally greater and the number of pixels demonstrating significant changes for a given region generally involved a greater volume than that seen with placebo response (
Table 2,
Figure 2).
Discussion
There are two key findings from this small but unique study of brain changes in glucose metabolism associated with placebo response. First, placebo response was associated with regionally specific changes in brain function. Second, while comparable brain changes were seen with both drug and placebo administration, drug response was not merely the same as the placebo effect, as active fluoxetine treatment was associated with additional and unique changes in the brainstem, striatum, and hippocampus. Despite these differences, clinical response to treatment, independent of whether the substance administered was active fluoxetine or placebo, was associated with a common pattern of reciprocal changes in specific cortical and paralimbic regions.
Response Mechanisms
Mechanisms of antidepressant medication response have generally focused on adaptive neurochemical changes, including long-term aminergic reuptake inhibition and associated presynaptic autoregulatory desensitization, up- and down-regulation of multiple postsynaptic receptor sites, and receptor-mediated second messengers and neurotrophic effects
(62–
64). Pharmacological studies have emphasized a bottom-up cascade; brainstem, limbic, and subcortical sites are generally viewed as the primary sites of drug action
(65,
66), with secondary cortical changes seen as secondary effects of long-term treatment
(67–
71).
Nonpharmacological antidepressant treatments, on the other hand, emphasize the alteration of relevant cognitions, affects, and maladaptive information processing caused by the clinical depression
(72–
75). Attention to development of new cognitive strategies that enhance awareness of self-defeating thinking styles and behavioral patterns that contribute to feelings of depression is a primary goal
(76–
80). These approaches can also be conceptualized as affecting changes in specific neural pathways by means of top-down or cortical mechanisms.
Placebo administration, while inarguably a nonpharmacological intervention, has certain distinctions from more explicit cognitive or psychotherapeutic strategies. From a cognitive perspective, placebo administration, unlike formal cognitive therapies, is an implicit form of antidepressant treatment
(1–
3). Also, there is an expectation that improvement will take place, but unlike cognitive or other psychotherapeutic approaches, there is no specific or explicit process or procedure involved. Moreover, placebo administration is also not a totally passive process. Patients in a placebo-controlled trial enter into a formal research relationship with a well-defined beginning and end, with the hope and expectation that their symptoms will improve over the course of the study
(81,
82). The therapeutic milieu of an inpatient psychiatric ward and the associated support system may be an additional factor
(83,
84). A “resetting” of normal social and physical rhythms disrupted by psychosocial stressors that contribute to the initiation and maintenance of a depressive state may be indirectly facilitated by an inpatient treatment trial that removes a patient from the often maladaptive environment in which his or her illness is perpetuated
(85,
86). It is therefore emphasized that administration of placebo is not absence of treatment, just an absence of active medication. These findings have important implications for more specific types of nonpharmacological therapies that explicitly target maladaptive cognitive processing. Clearly, this psychosocial and physical re-setting facilitated by the hospitalization itself was not adequate to initiate or maintain a clinical response in all patients in our study.
Fluoxetine-Specific Metabolic Effects
The unique brain changes in the brainstem and hippocampus seen in the group receiving active fluoxetine might be interpreted as medication effects responsible for side effects rather than as clinical response. Said another way, one might view the findings as supporting the premise that both groups showed a placebo response, with no added value of active drug. However, this does not appear to be the case, because responders and nonresponders receiving the active drug did not show comparable brain changes
(29). To the contrary, modification of hippocampal and brainstem metabolic changes seen early (1 week) in the course of treatment in all patients receiving active drug did not occur in the patients who showed no clinical improvement at 6 weeks. A drug-response interaction effect appeared necessary for treatment response, with the brainstem and hippocampus playing a fundamental role in mediating cortical and limbic effects
(63,
64). These time-course effects were discussed in detail in a previous article on this patient group
(29).
Time Course of Regional Changes
Also unanswered is whether the additional hippocampal, brainstem, striatal, and insula changes seen uniquely in drug-treated responders facilitates and maintains clinical response in the long term. It might be speculated that modulation of these regions results in the observed changes of greater magnitude in response-specific cortical and paralimbic regions, thus strengthening or stabilizing the newly established state of equilibrium over time
(64,
67–70). It is additionally hypothesized that absence of these changes in placebo responders may put this group at greater risk for relapse and recurrence. Clinical experience and research studies of relapse suggest that long-term response to therapy is better maintained with active drug than with placebo
(13). Mechanisms mediating chronic fluctuations in the disease state with anticipated recurrences, however, remain an open issue
(14–
16). Since the placebo responders, like the drug responders, did not have a complete remission by the end of the 6-week trial, all of the patients were offered active drug and individualization of treatment at the completion of the PET study. It is, therefore, not possible to determine if the brain metabolism changes were adequate to prevent relapse in those receiving placebo over time.
In this study, there were no significant demographic, clinical, or neuropsychological markers indicating propensity for placebo response, although the group was clearly very small
(87–
90). It was noted, however, that placebo responders, unlike drug responders, showed a common 6-week, response-specific increase in posterior cingulate metabolism at 1 week. While the overall 1-week metabolic pattern did not reach omnibus significance, this early change is noteworthy since with active fluoxetine, metabolism in this region
decreases at 1 week and then increases by 6 weeks of treatment in drug responders. The switch at 1 and 6 weeks does not occur in drug nonresponders
(29). Presence of this early response-specific change in placebo responders suggests an important early effect critical to facilitating more widespread changes seen with long-term administration.
The ability to up-regulate activity in the posterior cingulate (i.e., increase metabolism) may be an early indicator of the brain’s compensatory capacity. This speculation is supported by a recent report
(40) demonstrating comparable increases in metabolism in the posterior cingulate midway through a 12-week course of interpersonal psychotherapy. Known anatomical connections from the posterior cingulate to the identified cortical, limbic, and brainstem regions that are necessary for clinical response are one possible mechanism mediating these effects
(91–
93). Early increases in metabolism in the posterior cingulate may also prove to be a marker of drug responsivity, identifiable during the placebo wash-in phase of a clinical trial
(94–
96). Evidence that placebo nonresponders fail to show this 1-week posterior cingulate change would strongly support this hypothesis. There was inadequate power to test this hypothesis in the current study.
Other Considerations
The possibility of spontaneous remission as part of the natural course of a major depressive episode is also an issue in the evaluation of clinical response to any treatment intervention. In the present study, there was no way to determine whether spontaneous remission might have contributed to the effect for either the patients administered placebo or drug. The ideal control group to address this question—patients actively prescribed no treatment—were not studied
(97), as we did not consider this situation to be ethical. There were, however, no significant differences between groups with respect to episode duration before hospitalization (
Table 1). Statistical power was inadequate to examine time course differences in clinical response between the 1-week and 6-week PET scans. Nonetheless, even if some patients did have spontaneous recovery rather than a placebo or drug response, the common changes across groups would still support our hypothesis that symptom improvement requires changes in specific brain regions.
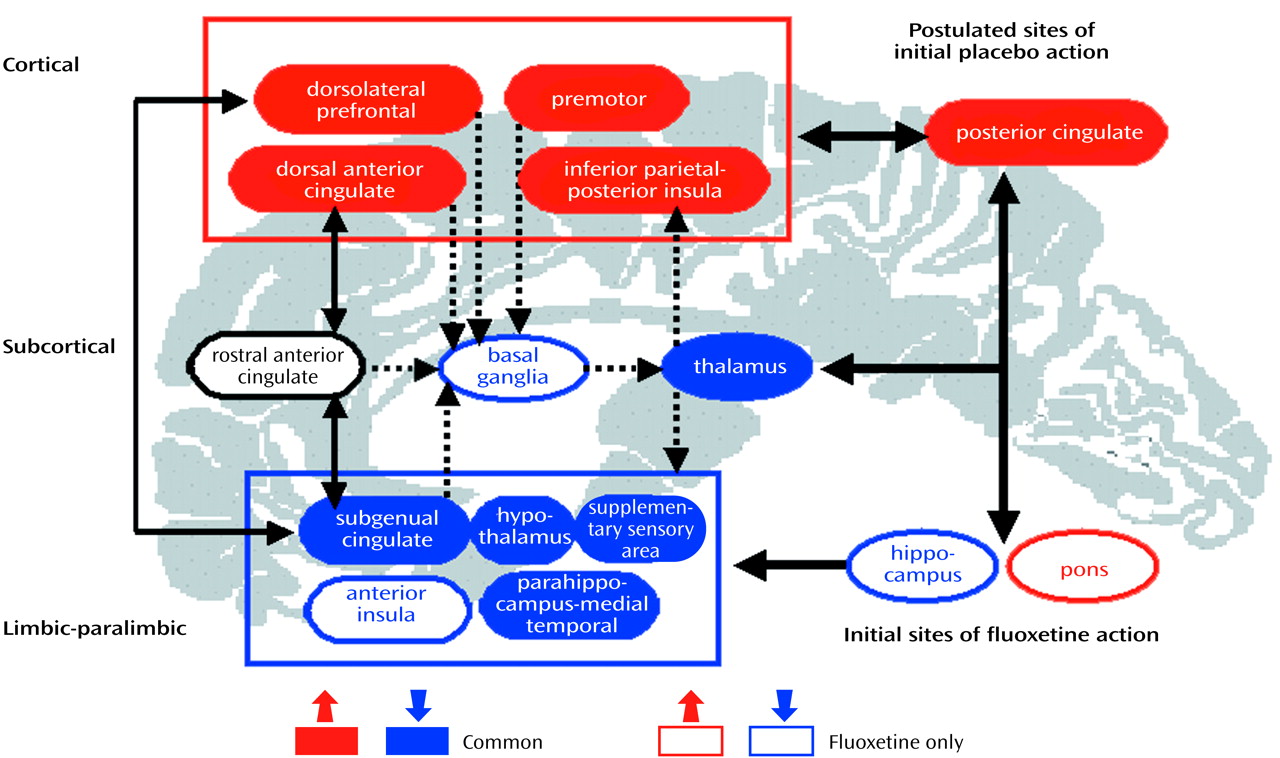
In conclusion, the combination of dorsal-cortical increases and limbic-paralimbic decreases in glucose metabolism, with response to both drug and placebo intervention, suggests that therapy for depression targeting either subcortical (brainstem) or cortical (frontal-posterior cingulate) sites should be equally effective if there is a preserved compensatory capacity in the obligatory circuit overall (
Figure 3). Progressively more aggressive treatments needed to ameliorate symptoms in some patients may reflect the poor adaptive capacity of this network. Similarly, the functional integrity of these pathways may explain the comparable clinical efficacy of pharmacological and cognitive treatments in randomized controlled trials conducted in patients with nonrefractory depression. The results of this study therefore suggest that facilitation of specific adaptive reciprocal limbic-cortical changes is necessary for depression remission, regardless of the mode of treatment
(98).