Psychiatry and neurology are rife with disorders suggesting focal hyperexcitability of the brain. An obvious example is focal epilepsy, which produces unregulated activation of neural groups that, at least on initiation, is localized to a specific region of the brain. Many other disorders in psychiatry and neurology are characterized by episodic behavioral and/or cognitive activation that suggests focal brain activation. These disorders include myoclonus, Tourette’s syndrome, hallucinations, and posttraumatic stress disorder (PTSD). Because of the limited efficacy of currently available clinical interventions, new tools to probe and treat brain hyperexcitability disorders would be highly desirable.
Repetitive transcranial magnetic stimulation (rTMS) offers a noninvasive method for altering excitability of the brain. This method uses an electromagnet placed on the scalp that generates magnetic field pulses of very short duration (100–300 msec) approximately 1.5–2.0 T in strength. Magnetic fields pass largely undistorted through the scalp and skull
(1,
2). Their rapid rise and fall induce corresponding electrical fields that stimulate small regions of the brain. The diameter of cortical tissue stimulated directly is approximately 2–3 cm
(3) and depends on the shape and configuration of the coil. Mathematical models predicted approximately 2-cm magnetic field penetration from the scalp surface
(4), which is sufficient to span gray matter for most cerebral cortical regions immediately adjacent to the skull. Some neuroimaging data
(5) suggest that TMS accesses deeper brain structures, including enfolded gray and neighboring white matter.
In this review we will consider published studies of rTMS administered to humans at a rate of 0.3–1 Hz. By convention, rTMS in this frequency range is referred to as “slow,” whereas “fast” rTMS refers to stimulation greater than 1 Hz. The studies of slow rTMS are reviewed in two sections. The first covers studies of normal subjects, and the second relates to patients with specific illnesses. In the third section we discuss long-term depression and long-term depotentiation as potential models for understanding the mechanism of slow rTMS.
Slow rTMS Studies of Normal Human Subjects
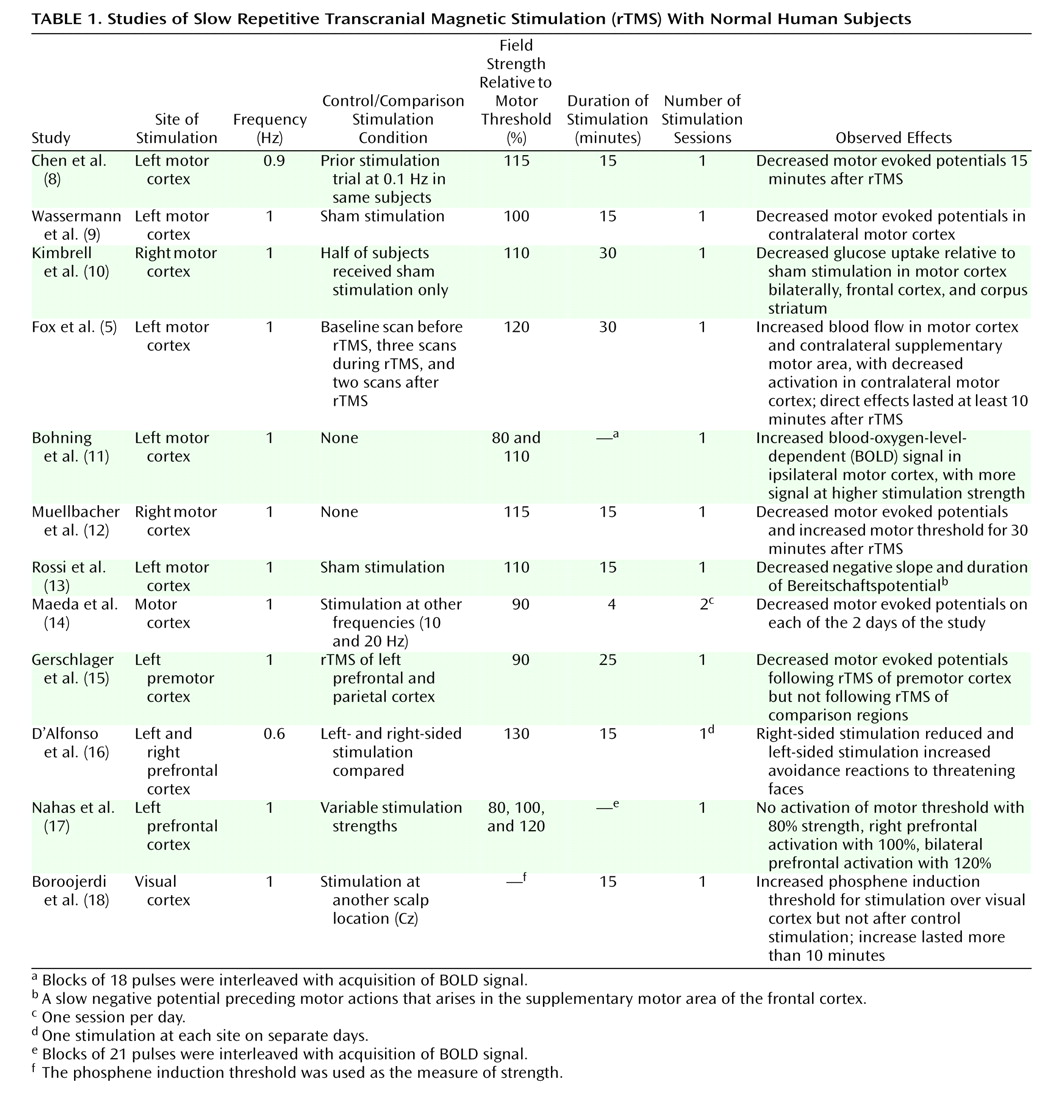
The majority of studies using slow rTMS have been directed at the primary motor cortex (
Table 1). The reason is simple—functional localization of stimulation is readily achieved by adjusting the site of rTMS so that specific peripheral muscle groups are activated. In contrast, rTMS delivered to the association cortex is largely silent, at least in terms of immediate effects, although higher-frequency (10–20 Hz) rTMS can elicit speech arrest or memory curtailment
(19,
20). Thus, it is more difficult to ascertain the functional “location” of rTMS delivered to regions other than the motor cortex in the absence of neuroimaging methods (see, for instance, the work of Paus et al.
[21]).
To our knowledge, the first study examining the physiological effects of slow rTMS on motor cortex and corticospinal signal propagation to skeletal muscle was reported by Chen et al.
(8). The amplitude of motor evoked potentials for the abductor pollicis brevis muscle in response to single TMS pulses to the primary motor cortex was measured at baseline and immediately after 0.9-Hz rTMS of 15 minutes’ duration. Significant reductions in motor evoked potentials were observed. These data likely do not reflect simple fatigue since fast rTMS delivered to the motor cortex enhances motor cortical excitability
(22). The findings of Chen et al.
(8) have been replicated by other groups, and suppressive effects on the size of motor evoked potentials have lasted up to 30 minutes after stimulation
(12,
14). Other studies, moreover, have demonstrated that the suppressive effects of slow rTMS can be propagated to other regions not directly stimulated, presumably by functional connections. For instance, slow rTMS of the left primary motor cortex reduces motor evoked potentials elicited by single-pulse TMS administered to the right primary motor cortex
(9), an effect presumably mediated by transcallosal projections. Similarly, slow rTMS delivered to the premotor cortex, another brain area projecting to the primary motor cortex, reduces motor evoked potentials elicited by stimulation of the latter
(15). Finally, slow rTMS of the primary motor cortex was found to diminish the Bereitschaftspotential
(13), a slow negative potential preceding motor actions that arises in the supplementary motor area of the frontal cortex. The supplementary motor area exchanges extensive projections with the motor cortex. Thus, slow rTMS delivered to different components of motor circuits appears to propagate to other circuitry components.
In terms of neuroimaging findings, Fox and colleagues
(5) found increased activation in the motor cortex, as assessed by H
2[
15O] positron emission tomography (PET), immediately after 1-Hz rTMS. Similarly, Bohning et al.
(11) demonstrated increased activation both locally and in distant sites by using functional magnetic resonance imaging (fMRI) interleaved with 1-Hz rTMS of the motor cortex. However, one study
(10) using [
18F]fluorodeoxyglucose PET showed reduced cortical metabolic activation during 1-Hz rTMS of the motor cortex.
Studies of slow rTMS in normal subjects delivered to brain regions other than the motor cortex have also been reported (
Table 1). Visual cortex excitability can be assessed by means of the TMS threshold for inducing visual phosphenes
(18). Slow rTMS to this region led to increased phosphene thresholds, suggesting decreased cortex excitability. In another study
(16), the effects of right and left prefrontal slow rTMS were compared in normal subjects in terms of their responses to angry faces during performance of a version of the Stroop task involving facial expression of emotions. Consistent with accounts of neural mechanisms of approach and withdrawal behaviors, right-sided stimulation reduced avoidance reactions to angry faces, while left-sided stimulation enhanced attentional responses to these stimuli. Acquisition of fMRI data interleaved with pulse sequences of 1-Hz rTMS delivered to the prefrontal cortex has been studied by Nahas et al.
(17). Stimulation produced activation of the prefrontal cortex that correlated with the strength of rTMS.
To summarize, the findings from studies that assessed neural reactivity to inputs (either from TMS itself or from neural sources arising from cognitive or motor activation) suggest strongly that slow rTMS reduces reactivity. However, functional neuroimaging studies that assessed the immediate effects of slow rTMS
(5,
11,
17) generally demonstrated increased cortical activation. The latter is not surprising since even single-pulse TMS activates neurons, as demonstrated by skeletal muscle contractions arising from motor cortex stimulation. These two sets of findings are not inconsistent, since elevated baseline activation of neural tissue does not preclude and may even lead to reduced reactivity to physiological or electromagnetic inputs.
Mechanism of Action of Slow rTMS
To our knowledge, only one study to date has directly examined the effects of slow rTMS directly on cortical tissue
(52). Rodent auditory cortex was studied by using a small device capable of delivering 1–2 seconds of magnetic stimulation in a highly localized fashion. Iterations of rTMS appeared to induce activating effects initially, which then evolved into sustained deactivation as assessed by the rate of evoked responses. Although the effects of rTMS with stimulation frequencies ranging from 1 to 10 Hz were studied, these effects were detected primarily at stimulation frequencies higher than 5 Hz. These data may not, however, be very relevant to rTMS studies in humans, since stimulation in humans is administered over much longer time periods (i.e., 15–30 minutes).
In keeping with the hypothesis of Post et al.
(6), in the remainder of this section we will consider studies of experimentally induced synaptic modification known as long-term depression. Long-term depression is induced by low-frequency electrical stimulation (1–5 Hz) directly applied to afferent fibers. Such stimulation results in long-lasting decreases in synaptic transmission. Enduring modifications in synaptic strength, perhaps analogous to long-term depression and long-term potentiation, are hypothesized to mediate information storage in the brain. Consistent with this view is the fact that hippocampal long-term potentiation can endure over many weeks in unanesthetized rabbits—indeed for as long as the animal preparation can be sustained—if repeated stimulation trains are administered
(53). Long-term depression has been observed in vitro in several rodent brain areas, such as the hippocampus, neocortex, striatum, amygdala, and cerebellum
(54,
55). In slices taken from the human temporal cortex, both long-term potentiation and long-term depression are inducible with the same stimulation characteristics used in the rodent studies
(56). In human neocortical slices long-term depression can be induced with low-frequency (1-Hz) 15-minute stimulation of layer IV afferents, and long-term potentiation can be induced with high-frequency stimulation (40–100 Hz). Furthermore, low-frequency stimulation is able to reverse high-frequency-induced potentiated synaptic responses, a phenomenon referred to as “depotentiation,” whereby synaptic weights are “reset” to baseline levels.
The fact that human neocortical synapses exhibit neuronal plasticity in response to high- and low-frequency electrical stimulation similar to that exhibited in rodent studies strengthens the hypothesis that these events contribute to naturally occurring human neuroplasticity. The credibility of long-term depression as a model of human neuroplasticity was initially questioned because of difficulties in inducing this phenomenon reliably in intact adult animals. More recent work demonstrated, however, that long-term depression is indeed inducible with traditional 1-Hz stimulation in the hippocampus and cortex of freely moving rats, and once induced, it can last for several days
(57–
59).
There are many parallels between the slow rTMS administered to humans and the low-frequency electrical stimulation used to induce long-term depression. Both phenomena seem to obey similar frequency-dependent relationships. Low-frequency direct electrical stimulation (1–10 Hz) usually diminishes synaptic efficacy such that the magnitude and duration of long-term depression decrease as stimulation frequency increases from 1 Hz up to 10 Hz
(60,
61). Analogously, cortical excitability following rTMS is decreased within that same frequency range
(14). Motor cortex excitability following rTMS has been observed to be more consistently suppressed at 1-Hz stimulation than at frequencies higher than 5 Hz
(14,
62). Higher-frequency stimulation (>10 Hz) produces increased synaptic efficacy after direct electrical stimulation
(60) and increased cortical excitability after rTMS
(22). Furthermore, both low-frequency electrical stimulation and slow rTMS can induce transsynaptic effects. Induction of long-term depression in the sensorimotor neocortex of the awake rat is accompanied by alterations on the contralateral side that resemble long-term depression, although these alterations are of shorter duration
(59). As already discussed, 1-Hz rTMS has been shown to propagate across different components of motor circuits
(9,
13,
15). In addition, the duration of stimulation required to reduce cortical excitability by slow rTMS (see reference
31) is similar to the duration of low-frequency electrical stimulation needed to induce long-term depression (approximately 15 minutes).
Neocortical long-term depression and changes in the cortical excitability induced by slow rTMS appear to accumulate in an additive fashion as the number of stimulations is increased over many days. Although in vivo hippocampal long-term depression is inducible with one session of stimulation, stable neocortical long-term depression requires that the stimulation is spaced and repeated over several days
(59). In the sensorimotor cortex of freely moving rats, a single-session stimulation of the white matter with 1 Hz for 15 minutes leads to long-term depression lasting only hours, whereas daily stimulation for 10 days can induce long-term depression lasting for at least 2 weeks
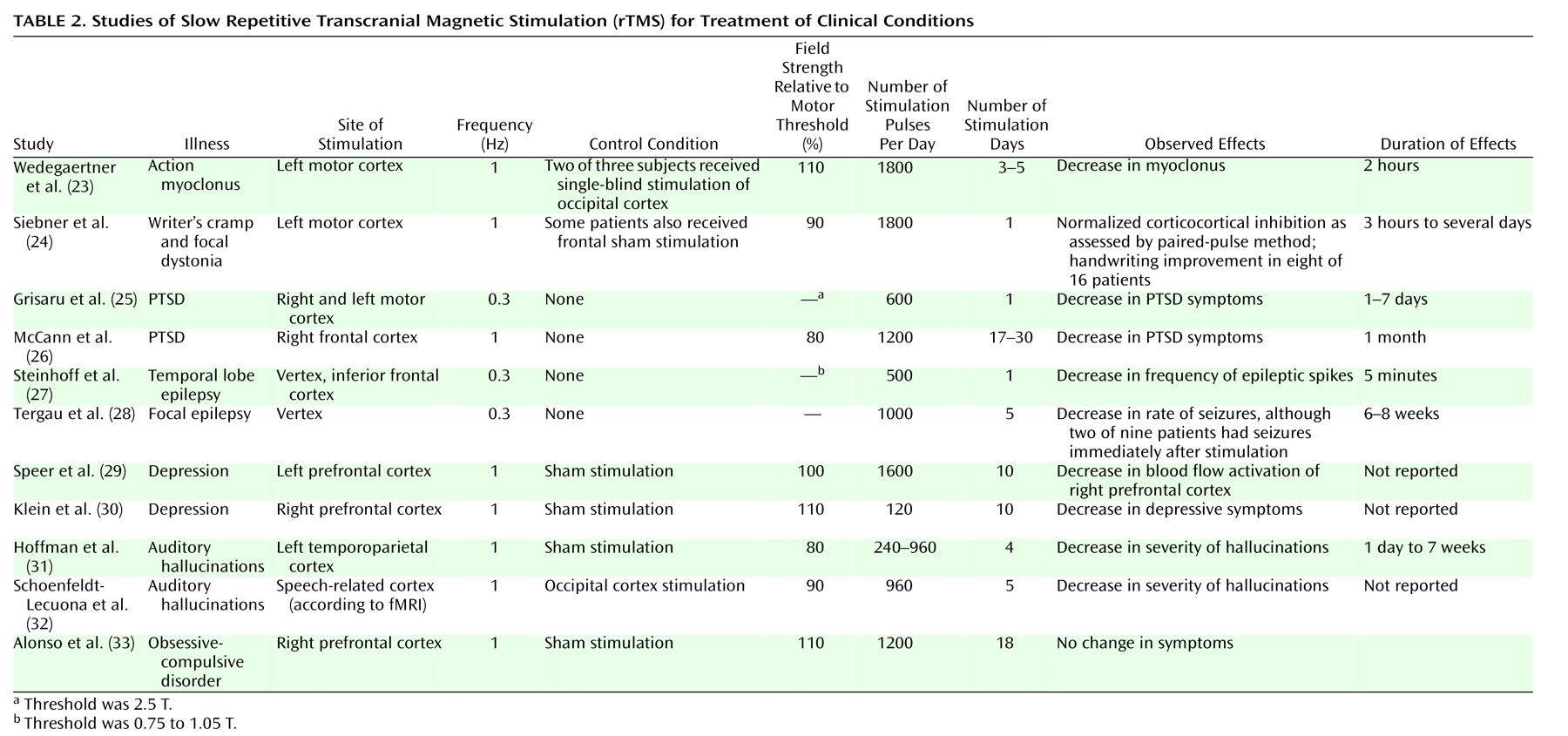
(59). A systematic study comparing the duration of changes in cortical excitability following one session of rTMS with the duration after multiple daily rTMS sessions is lacking. However, neuropsychiatric studies of enduring effects on mood have generally required several instances of stimulation on separate days (
Table 2). In our more recent study of patients with auditory hallucinations, we found large increases in the duration of symptom improvement when the number of “doses” of extended stimulation (i.e., lasting 16 minutes) was increased from one to seven (unpublished 2002 study). Parallel trends in studies of PTSD and epilepsy have also emerged (
Table 2). Thus, studies of both slow rTMS and long-term depression suggest additive efficacy when higher numbers of spaced, daily stimulations are administered.
The reversal, or depotentiation, of previously enhanced synaptic transmission due to long-term potentiation may be the most relevant model for slow rTMS when used as a therapeutic tool. Human neural networks can undergo depotentiation, as is seen with 1-Hz electrical stimulation of already potentiated synapses in slices from the human temporal cortex
(56). In rodent in vivo models, depotentiation of hippocampal long-term potentiation can be induced with low-frequency electrical stimulation, but only within a relatively short interval after induction of long-term potentiation
(63,
64). Neocortical long-term potentiation seems to be more amenable to depotentiation, even several days after its induction. In one investigation
(59), single-session low-frequency electrical stimulation of white cortical matter reversed potentiated synaptic responses that had been established for 8 days. Thus, long-term depotentiation sustained over many days may be more achievable than long-term depression.
In awake cats, 1-Hz stimulation of the amygdala caused a depotentiation that lasted for several days, with synaptic efficacy returning to a potentiated state roughly 70 days later
(65). An “experimental pathology” studied with low-frequency electrical stimulation is kindled seizures. Daily 1-Hz electrical stimulation of the amygdala for 15 minutes over 1 week succeeded in suppressing kindled seizures in this brain region for 21 days
(66). Low-frequency stimulation in this study did not disrupt any ongoing behavior, suggesting again that this manipulation may have selectively reversed pathologically enhanced excitation associated with the kindled response. Similarly, in the study of writer’s cramp by Siebner et al.
(24), alterations in inhibitory circuits following slow rTMS—as demonstrated by paired-pulse testing, the post-TMS silent period, and handwriting pressure—were elicited only in patients with dystonia (not in patients with simple writer’s cramp). No changes in laboratory measures were noted in the normal comparison subjects. In our study of auditory hallucinations (unpublished 2002 study), there was a high rate of improvement of the hallucinations but no impairment in results on neuropsychological tests. One possible explanation is that slow rTMS selectively depotentiates enhanced synaptic weights associated with pathological states while leaving baseline weights unchanged.
It would be difficult to assert, however, that slow rTMS in human subjects causes long-term depression or depotentiation in the underlying cortex or its projections under all conditions. In animal studies using electrical stimulation of specific pathways, low-frequency electrical stimulation does not always cause long-term depression or depotentiation. It seems that specific frequencies induce distinct forms of synaptic plasticity in different synapses. Low-frequency electrical stimulation (0.5–10 Hz) causes enduring long-term depression in area CA1 of the hippocampus and in some neocortical areas
(55,
60). Paradoxically, low-frequency electrical stimulation induces long-term potentiation in the prelimbic cortex and in CA1-subiculum synapses, the hippocampal output pathway to the cortex
(67). Conversely, in a study of synapses arising from mossy fiber projections to the CA3 region of the hippocampus
(68), high-frequency stimulation (100 Hz) resulted in long-term depression, while stimulation at 1–3 Hz had no effect. Long-term depression for this class of synapses was obtainable only with specific paired stimulation of two converging pathways, the commisural pathway and the mossy fibers. Even neighboring synapses follow different plasticity rules. In the dentate gyrus, 400-Hz stimulation to the lateral perforant pathway induced long-term potentiation in the synapses of the same pathway while inducing long-term depression in the neighboring medial perforant pathway
(69). These findings arise from the fact that electrical stimulation studies are very selective, usually involving activation of a specific fiber pathway. In contrast, rTMS employs a coil applied to the scalp that activates a relatively large area of heterogeneous synapses and fiber pathways. Some of the affected pathways may be already potentiated, others depressed, and some may remain unchanged. A magnetic field pulse applied to a large area with propagated effects in even more distant areas is likely to produce different effects on different synapses depending on their synaptic weight at the time of stimulation, with far less specific effects than those produced by electrical stimulation.
The heterogeneous and relatively nonselective effects of rTMS may account for the heterogeneous responses or individual variability observed in humans
(29,
70). In some scenarios, this may be an advantage in the sense that 1-Hz stimulation may depotentiate only the pathologically potentiated synapses while leaving intact the nonpotentiated (baseline) synapses. Nonetheless, it seems clear that a simple model of slow rTMS based on long-term depression or long-term depotentiation cannot account for the extraordinary complexity of relevant cortical network physiology.
Genetic background and behavioral state affect the induction of long-term depression in awake animals
(58,
61,
71). Only certain rat strains manifest lasting hippocampal depression; other strains receiving the same low-frequency electrical stimulation do not
(61). Furthermore, larger and more lasting hippocampal depression is observed in rats exposed to behavioral stress
(71) or novel environments
(58). Other behavioral factors, such as the level of arousal, attention, and fatigue, may also influence the induction of long-term depression. Similarly, substantial individual variability with rTMS stimulation in human studies has also been reported
(70). It may be important, therefore, to consider the genetic makeup and behavioral state of subjects in assessing outcomes of rTMS treatment.
Closing Comments
The results of this review suggest that slow rTMS has considerable promise as a tool for probing normal and abnormal brain function and may even lead to new therapeutic tools. A range of studies are indicated on the basis of the reports we have outlined.
First, additional slow rTMS studies of normal subjects involving brain areas other than the motor cortex are needed. One especially neglected area is assessment of delayed or sustained effects of slow rTMS in terms of neural reactivity to cognitive or external inputs. This area is especially relevant to therapeutic applications of slow rTMS that by definition require effects that are sustained. We know of one neuroimaging study of depressed patients
(29) that assessed delayed effects of 1-Hz rTMS 3 days after completion of the protocol, and it showed reduced cortical activation. Similarly, the PTSD study by McCann et al.
(26) showed reduced metabolic activation 90 minutes and 24 hours after completion of slow rTMS trials in two patients. These findings are consistent with clinical, behavioral, and motor studies suggesting sustained diminution of neural reactivity after slow rTMS. In contrast, neuroimaging studies that assessed immediate effects demonstrated increased activation after 1-Hz rTMS
(5,
11,
17). The relationship between immediate and sustained or longer-term effects of slow rTMS needs further clarification. It is possible, for instance, that immediate activating effects of cortical neurons after slow rTMS accompany or lead to long-term depotentiation of pathologically reinforced neurocircuitry projections to these neurons.
Second, characterization of the indirect, propagated effects of slow rTMS is also critical. Neuroimaging data for patients with depression and epilepsy, for instance, have suggested that greater suppressive effects of 1-Hz rTMS are obtained in the cortical region contralateral to that being stimulated
(29,
46). If indirect (i.e., propagated) effects of rTMS are distinct from direct effects, this finding would be important in designing intervention studies based on known cortical patterns of pathological activation/deactivation.
Third, intervention studies involving disease conditions need to include larger numbers of subjects and to have rigorous control conditions. The effects of varying stimulation intensity, duration, and location should be assessed. These studies are likely to be enhanced by study designs that are guided by the known regional pathophysiology of the disorder in question. It may be possible to enhance intervention outcomes by using patient-specific neuroimaging data to position the stimulation coil for therapeutic trials
(32).
Fourth, there is a need for neurophysiological studies in animals that delineate the mechanism of action of slow rTMS in terms of the behavior of single neurons and alterations at the subcellular, molecular level. Of particular interest will be studies assessing whether scalp-administered slow rTMS is able to produce long-term depotentiation of previously established long-term potentiation. The possibility that long-term potentiation is a model for pathophysiology is relatively unexplored
(72) but consistent with the enduring nature of these experimental effects.
These research efforts, considered together, could provide important tools for probing and possibly treating a broad range of neuropsychiatric disorders expressing focal brain hyperexcitability.