There has been a growing appreciation of the role of the basal ganglia in cognitive as well as in motor functioning. Discrete motor and cognitive circuits have been described that anatomically link the frontal lobe to the basal ganglia in parallel feedback loops. The striatal nuclei (caudate, putamen, and nucleus accumbens) serve as the “input nuclei” for the basal ganglia and nonreciprocally receive cerebral cortical input. Under the modulation of midbrain dopamine neurons, these nuclei project to the “output nuclei” of the basal ganglia, the globus pallidus, interna and substantia nigra, pars reticulate that, in turn, project to target thalamic nuclei. The frontal-basal ganglia feedback loops are completed by thalamic projections back to the cerebral cortex
(1–
3).
The basal ganglia, situated between the cortex and thalamus, modulate cerebral cortical functioning through these basal ganglia-thalamocortical feedback loops. More specifically, three prefrontal “cognitive/limbic” circuits have been proposed that are believed to be important for higher cognitive and limbic functions. Two of these circuits originate in the dorsolateral prefrontal cortex and in the lateral orbitofrontal cortex, respectively, and a third “limbic” circuit originates in the anterior cingulate gyrus
(1,
2). The striatum mediates procedural memory
(4); however, because of the heavy connection to the caudate nucleus from the dorsolateral prefrontal cortex
(5), it has also been postulated that the caudate nucleus is involved in working memory, a postulate supported by glucose utilization and functional magnetic resonance imaging (MRI) activation studies in primates
(5) and humans
(6). The caudate nucleus (with the nucleus accumbens) is the focus of this study because it is the primary target of the “cognitive/limbic” association cortex, whereas the putamen is the primary target for the sensorimotor cortex
(2). Furthermore, it has been proposed that the interaction between glutamate and dopamine at the level of the striatum, which in turn influences thalamic filtering, has great importance for understanding both psychomotor activity and psychosis
(7).
It is worth emphasizing that although the striatum receives input from widespread parts of the cortex, the frontal lobe is its main source
(8), and, according to Graybiel
(9), “much of the output of the system” from the basal ganglia is directed to the frontal cortex. Therefore, pathology in the caudate nucleus, the principal striatal recipient of prefrontal, nonmotor cortical input, might result in similar neurobehavioral deficits as pathology in the prefrontal, nonmotor cortex itself
(10). Indeed, there are now considerable empirical data supporting this hypothesis, including human neurological lesion data
(10), animal studies
(11), and studies of degenerative diseases affecting the caudate nucleus, including Huntington’s chorea and neuroacanthocytosis, that manifest behavioral and intellectual symptoms similar to those of patients with frontal lobe lesions
(8).
Both functional and structural neuroimaging studies of subjects with schizophrenia have pointed to abnormalities in the basal ganglia. Positron emission tomography (PET) studies have reported diminished striatal metabolic rates in never-medicated subjects with schizophrenia
(12). PET studies have also reported that haloperidol and fluphenazine, but not clozapine, increase metabolic rates in the basal ganglia of subjects with schizophrenia
(13,
14). Structural neuroimaging studies
(3,
15,
16) and some
(17,
18) but not all
(19) postmortem studies have reported enlarged basal ganglia structures in schizophrenia. Neuroleptic medication, however, clearly affects the morphology of the striatum
(20) and is likely an important confound. More recent studies of neuroleptic-naive subjects experiencing their first episode of schizophrenia or schizoaffective disorder, conversely, have shown reduced caudate volume suggestive of an intrinsic abnormality in the caudate nucleus
(21,
22).
In this study, we have chosen to measure the caudate nucleus in schizotypal personality disorder because individuals with schizotypal personality disorder, who have less severe psychotic symptoms and neuropsychological deficits than do those with schizophrenia
(23), do not require neuroleptic treatment or recurrent hospitalization, which avoids the confounds of medication and chronicity. In our study, we included only subjects with schizotypal personality disorder who were neuroleptic naive. Furthermore, genetic studies support the suggestion that schizotypal personality disorder may be in the schizophrenia spectrum
(24,
25), suggesting that pathology found in schizotypal personality disorder has implications for pathology in schizophrenia.
We measured the caudate nucleus bilaterally and, in an exploratory approach, also divided it into its head and body for separate analyses. We isolated the head of the caudate (which, as we defined our region of interest, includes the nucleus accumbens) because it is the most anterior part of the caudate nucleus and, hence, receives its major innervation from the more anterior part of the cortex; namely, the three regions of the prefrontal cortex described earlier in this article
(1). We hypothesized that both the total caudate nucleus volume and the volume of the head of the caudate nucleus would be smaller in neuroleptic-naive subjects with schizotypal personality disorder than in normal comparison subjects. Furthermore, we hypothesized that diminished caudate volume would correlate with poorer “frontal” neuropsychological functioning and with more negative, or abulic, symptoms. Finally, we measured lateral ventricular volume to determine whether a change in its size may have indirectly affected caudate volume, e.g., ventricular expansion might compress contiguous caudate structures, or, conversely, smaller caudate volume might result in larger ventricular volume.
Method
Subjects
Fifteen neuroleptic-naive male subjects with schizotypal personality disorder and 14 normal comparison male subjects, all right-handed, underwent MRI scanning. Subjects were recruited and diagnosed as described in detail in our previous report
(26). Briefly, subjects with schizotypal personality disorder were recruited from the community through newspaper advertisements. An initial telephone screening reduced the number of subjects from 303 to 84 right-handed men. Inclusion criteria were 1) age between 18 and 55 years; 2) English as the primary language; 3) no history of neurological disorder (including head trauma with loss of consciousness greater than 2 minutes); 4) no history of ECT, no drug or alcohol dependence ever, and no abuse in the last year; and 5) no current use of psychotropic medications (including steroids). Follow-up videotaped interviews of these subjects with the Structured Clinical Interview for DSM-IV—Patient Edition (SCID-P)
(27) and the Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II)
(28) were conducted by either a research psychiatrist (C.C.D.) or a research psychologist (M.M.V.). Interrater reliability (established by a second research psychologist [L.J.S.] reviewing videotapes) for the diagnosis of schizotypal personality disorder, as previously reported
[26], was high (kappa=0.89, N=25).
Thirty of the 84 subjects met DSM-IV criteria for schizotypal personality disorder, but 15 of these men were not included in the analysis: nine were lost to follow-up, three were claustrophobic and could not go in the MRI scanner, two exceeded the weight limit for the MRI scanner, and one was removed from the study because of technical problems with the MRI scan. The subjects who were not scanned did not differ in terms of demographic characteristics (age, education, estimated IQ, parental or personal socioeconomic status) from those who were scanned.
The subjects with schizotypal personality disorder met criteria for a number of comorbid personality and axis I disorders, including paranoid (N=6) and borderline (N=5) personality disorders, depression (N=2), dysthymia (N=1), panic disorder (N=1), alcohol abuse (N=1), and polysubstance abuse (N=1); all substance abuse occurred more than 2 years before testing. More information concerning the subjects’ characteristics is available elsewhere
(26).
Comparison subjects also were recruited from the community through newspaper advertising, and these men also were interviewed with the SCID-P and SCID-II. Comparison subjects had the additional requirements of not having a personal or family (first-degree relatives) history of major mental illness or a personal history of personality disorder.
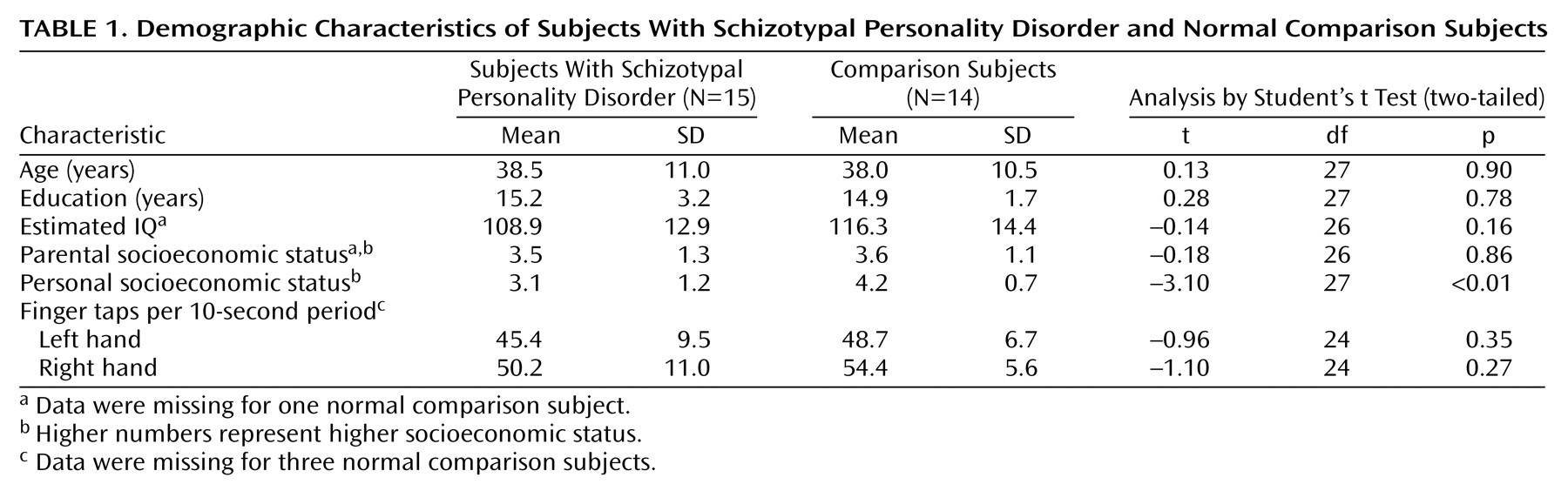
The mean age of the subjects with schizotypal personality disorder did not differ from that of the comparison subjects (38.5 years, SD=11.0, versus 38.0 years, SD=10.5). There were also no significant group differences in other demographic characteristics, including number of years of education completed, estimated IQ, and parental socioeconomic status; however, the groups differed in personal socioeconomic status (
Table 1).
Measures of Psychopathology
All subjects underwent a standard neuropsychological test battery as described elsewhere
(23). These included a delayed alternation spatial working memory task
(29) and the Controlled Oral Word Association Test
(23), a verbal fluency working memory task. Subjects with schizotypal personality disorder were assessed further with the Scale for the Assessment of Negative Symptoms (SANS)
(30) and the Scale for the Assessment of Positive Symptoms
(31). Scores for total number of positive and negative symptoms were derived from a composite score of the nine individual clinical items from DSM-IV for schizotypal personality disorder, divided into five positive and four negative symptom clusters, on the basis of the SCID-II personality disorder interviews. We also measured left- and right-hand finger tapping as an index of speed and dexterity of motor performance. Written informed consent was obtained from all subjects after a complete description of the study.
MRI Methodology
Image acquisition and postprocessing
The images were acquired by using a 1.5-T General Electric scanner (GE Medical Systems, Milwaukee). The MRI methodology has been described elsewhere
(26). Briefly, the acquisition protocol involved two MRI pulse sequences. In the first acquisition protocol, a three-dimensional Fourier transform spoiled gradient-recalled acquisition sequence was used that yielded a coronal series of contiguous 1.5-mm images throughout the brain. The following parameters were used for the spoiled gradient-recalled images: echo time (TE)=5 msec, repetition time (TR)=35 msec, repetition=1, nutation angle=45°, field of view=24 cm, acquisition matrix=256×256×124, voxel dimensions=0.9375×0.9375×1.5 mm. This protocol resulted in high-spatial-resolution images with excellent gray-white matter contrast, and it was used for measuring by means of manual tracing of specific caudate regions of interest. In the second acquisition protocol, a double-echo spin-echo sequence was employed. This yielded 108 contiguous 3-mm-axial double-echo (proton-density- and T
2-weighted) slices, with 54 levels, throughout the brain. The imaging parameters used were TE=30 msec, TR=3000 msec, field of view=24 cm, acquisition matrix=256×256, voxel dimensions=0.9375×0.9375×3 mm.
To measure intracranial contents and lateral ventricular CSF volume, the double-echo spin-echo axial slices were reformatted and coregistered to the coronal images obtained by 1.5-mm spoiled gradient-recalled acquisition. The resulting image was then used to generate whole brain segmentations into gray and white matter and CSF by using an iterative expectation-maximization segmentation protocol developed by Wells et al.
(32). The derived segmented images of the whole brain were then manually edited where necessary, for example, to separate left from right lateral ventricle or to demarcate the outer extent of the intracranial contents from skull and scalp tissue. All MRI measurements were performed blind to the subjects’ diagnostic status.
To reduce flow-related artifacts and to obtain low arterial-signal intensity, gradient moment nulling and presaturation of a slab inferior to the head were performed in both coronal and axial acquisitions. An anisotropic diffusion filter for both spoiled gradient-recalled and proton/T
2-weighted images was used to reduce noise before the processing of each set of scans as described elsewhere
(33). An algorithm for summing voxels of each tissue class (across slices) was also used to compute volumes.
Caudate nucleus and lateral ventricular regions of interest: anatomical landmarks
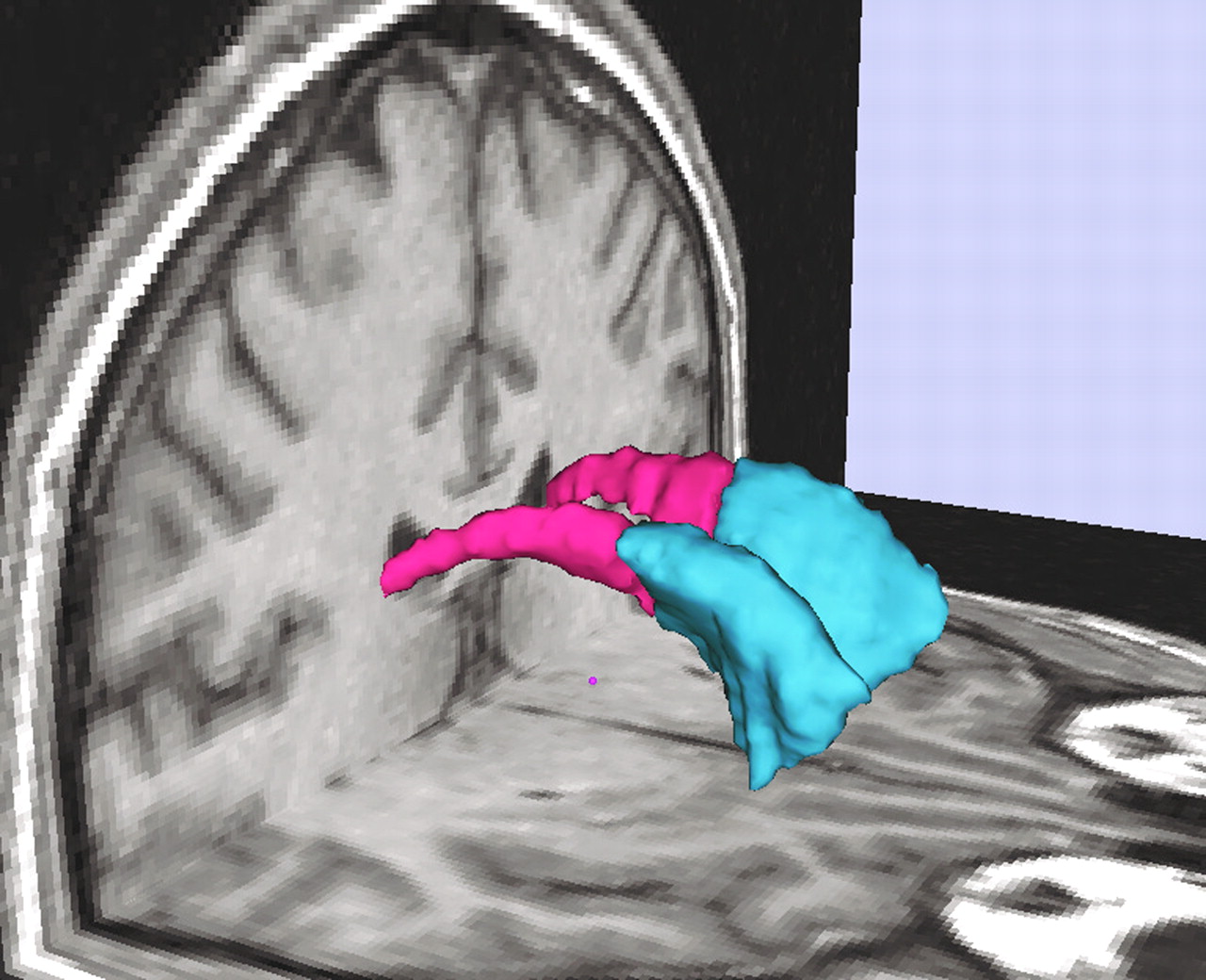
The caudate nucleus was measured bilaterally on all coronal slices in which it appeared. The head, body, and tail portions were included up to the point where the tail curved ventrally to border the lateral aspect of the atrium of the lateral ventricles. The anterior coursing of the tail was excluded because it was indistinct as a result of partial voluming (
Figure 1). Because of difficulty separating the nucleus accumbens (ventral striatum) from the merged caudate and putamen nuclei anteriorly, a vertical line was drawn from the most ventral point of the internal capsule inferiorly to the external capsule and was considered to be the lateral bound of the caudate nucleus
(3). We also parcellated the caudate nucleus into its head (anterior caudate nucleus) and body (posterior caudate nucleus), using the interventricular foramen of Monroe, bilaterally, as the anatomical landmark for this division. This foramen was defined, bilaterally, by the most posterior coronal slice where any part of the anterior column of the fornix could still be visualized. Our posterior boundary, for this parcellation, was the coronal slice prior to the atrium. We derived lateral ventricles as described, using whole-brain segmentation methodology. We measured the lateral ventricles only on those slices where they appeared contiguous to the head and body of the caudate nucleus.
Interrater reliability for whole left and right caudate nucleus volume was high (r>0.98); interrater reliabilities were also high for left (r>0.82) and right (r>0.87) anterior and left (r>0.94) and right (r>0.92) posterior caudate nucleus regions of interest. Interrater reliabilities, based on intraclass correlation coefficients, were computed by three raters on the brain scans of five subjects with schizotypal personality disorder and five normal comparison subjects who had been randomly selected from the total pool of subjects. Each rater independently traced every other slice for both left and right anterior and posterior caudate nuclei. Since the caudate extended anteriorly to posteriorly over approximately 42 1.5-mm coronal slices, this resulted in each rater tracing about 21 slices in each of 10 scans (hence, more than 200 slices per rater).
Statistical Analyses
Statistical analyses for MRI structural measures were performed on relative brain volumes to correct for variations in head size. Relative volumes were obtained by dividing absolute volumes by total intracranial contents and multiplying by 100. The effects of laterality alone (on the caudate nucleus and lateral ventricles) and then laterality plus region (anterior versus posterior) on the caudate nucleus were examined by repeated-measures analysis of covariance (ANCOVA) with age as a covariate, group (subjects with schizotypal personality disorder versus comparison subjects) as the between-subject factor, and laterality (left versus right) or laterality plus region (anterior versus posterior caudate) as the within-subject factors. When a main effect for group was found, follow-up planned Student’s t tests, with significance set at p<0.05 (two-tailed), were used to test the mean difference between groups in relative volumes of each caudate or lateral ventricular region of interest. Similar analyses of absolute brain volumes covarying for intracranial contents and age were also performed. Because of the relatively small number of subjects with schizotypical personality disorder in our study (N=15), and to avoid being unduly influenced by outliers, we used nonparametric Spearman correlations, with two-tailed p values, for all within-group correlations between caudate region of interest relative volumes and measures of psychopathology.
Results
Caudate Nucleus and Lateral Ventricular Volumes
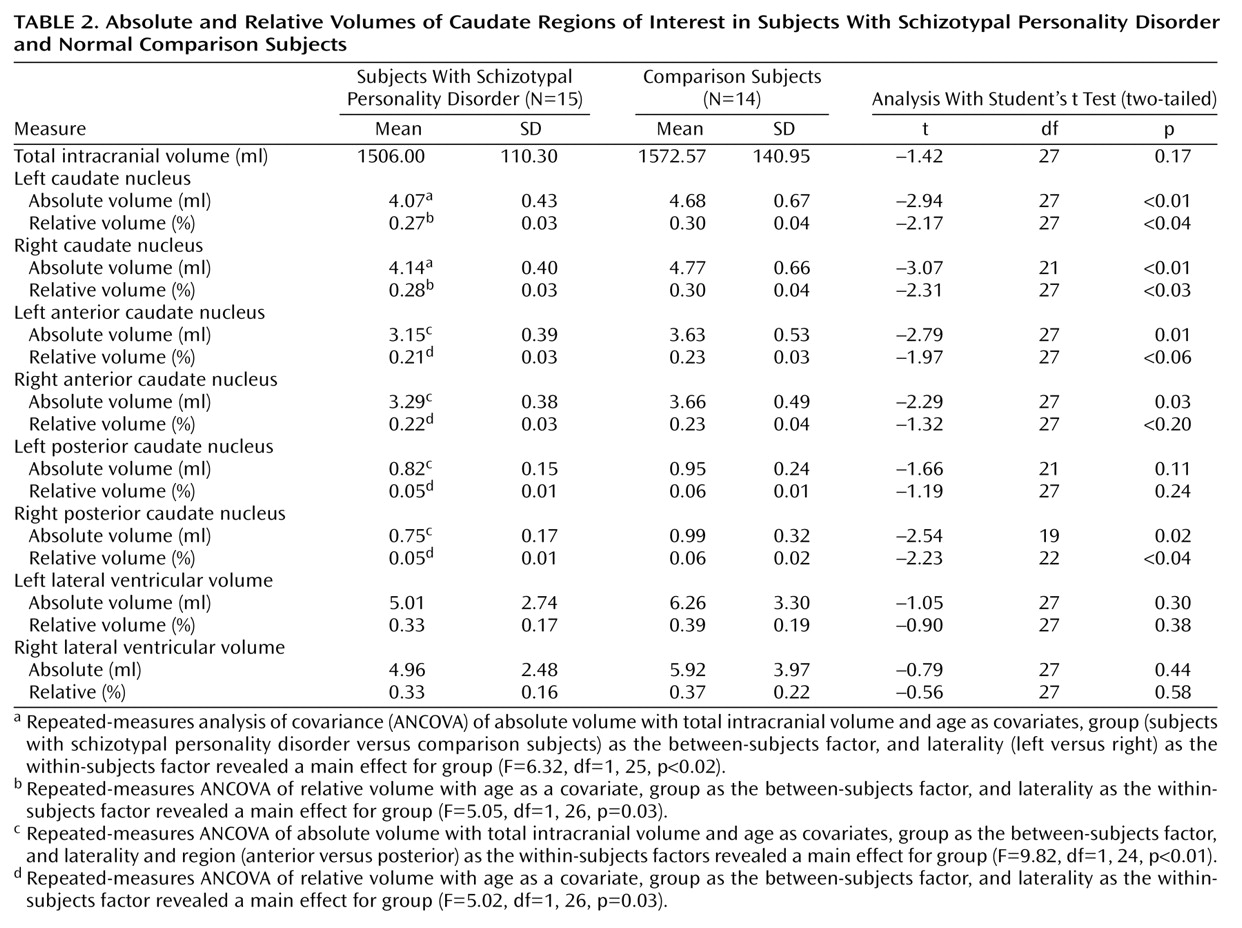
As shown in
Table 2, statistical tests of differences in region of interest volumes were performed on absolute and relative volumes. There were no group differences between subjects with schizotypal personality disorder and normal comparison subjects in total intracranial volume (t=–1.4, df=27, p=0.17) or in left (t=–0.90, df=27, p=0.38) or right (t=–0.56, df=27, p=0.58) relative lateral ventricular volume. Repeated-measures ANCOVA of caudate relative volumes with age as a covariate revealed a significant group difference (
Table 2). Follow-up planned contrasts applying Student’s t tests showed that right (t=–2.3, df=27, p<0.03) and left (t=–2.2, df=27, p<0.04) caudate relative volumes were significantly smaller in subjects with schizotypal personality disorder than in normal comparison subjects. The results of statistical analyses performed on absolute volumes fully paralleled those for relative volumes (
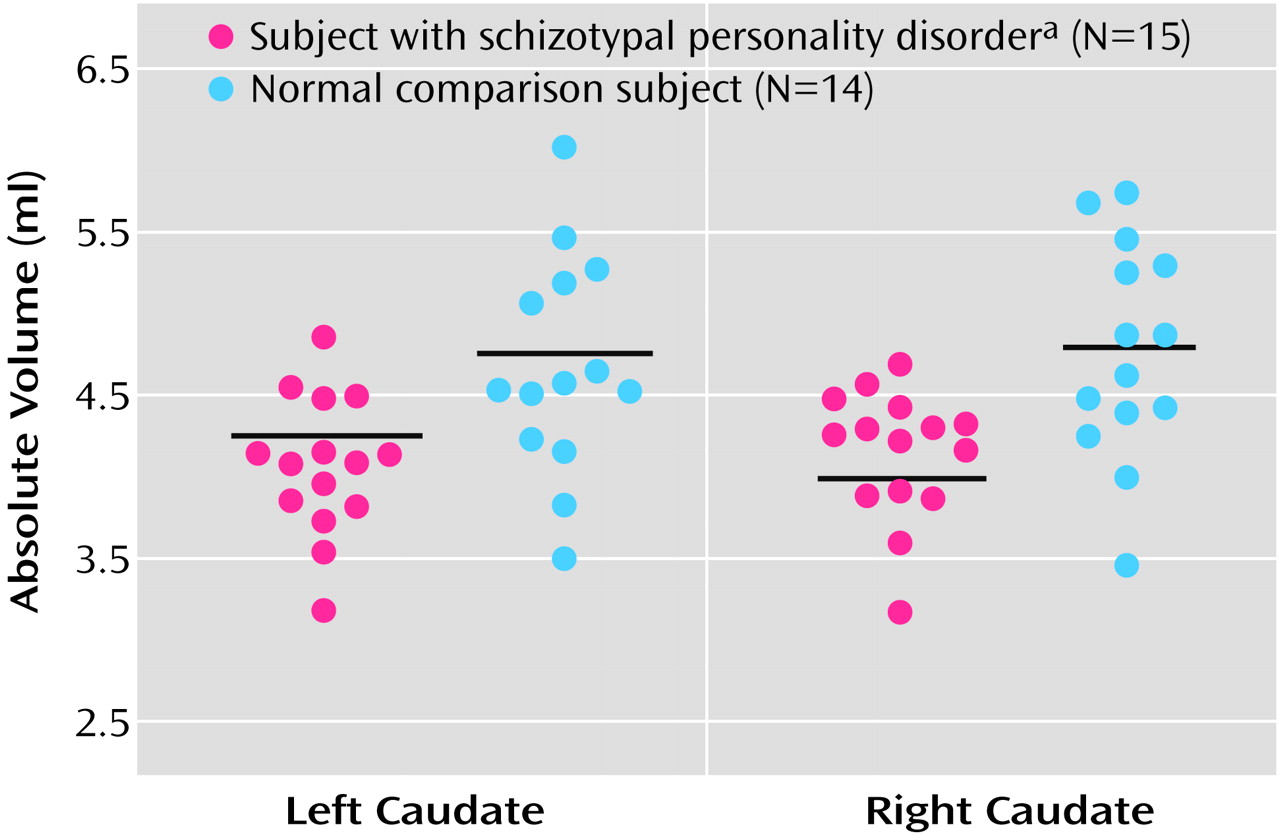
Table 2 and
Figure 2). In percentage terms, basing the calculations on volume values brought out to three places after the decimal, we found that the left and right caudate nucleus relative volumes in the subjects with schizotypal personality disorder were 9.1% and 9.2% smaller; absolute volumes were 13.1% and 13.2% smaller.
In an exploratory analysis of the caudate nucleus, parcellated into anterior and posterior regions, repeated-measures ANCOVA revealed a significant group difference in relative volumes (F=7.14, df=1, 25, p=0.01). Follow-up contrasts applying Student’s t tests did not yield significant differences for the left (t=–2.0, df=27, p<0.06) or right (t=–1.3, df=27, p<0.2) anterior caudate relative volume but did yield a significant difference for the right (t=–2.2, df=22, p<0.04) but not for the left (t=–1.2, df=27, p=0.2) posterior caudate relative volume (
Table 2). Repeating the same analysis for parcellated caudate regions of interest and covarying for intracranial contents and age yielded similar results for absolute volumes. In percentage terms, basing the calculations on volume values brought out to three places after the decimal, we found that the left and right anterior caudate nucleus relative volumes were smaller in subjects with schizotypal personality disorder by 9.1% and 6.4% and absolute volumes were 13.2% and 10.2% smaller. We did not find significant group differences for left or right lateral ventricular relative volume or absolute volume (0.30≤p≤0.58) (
Table 2).
Correlation Between Caudate Nucleus Volumes and Measures of Psychopathology in Subjects With Schizotypal Personality Disorder
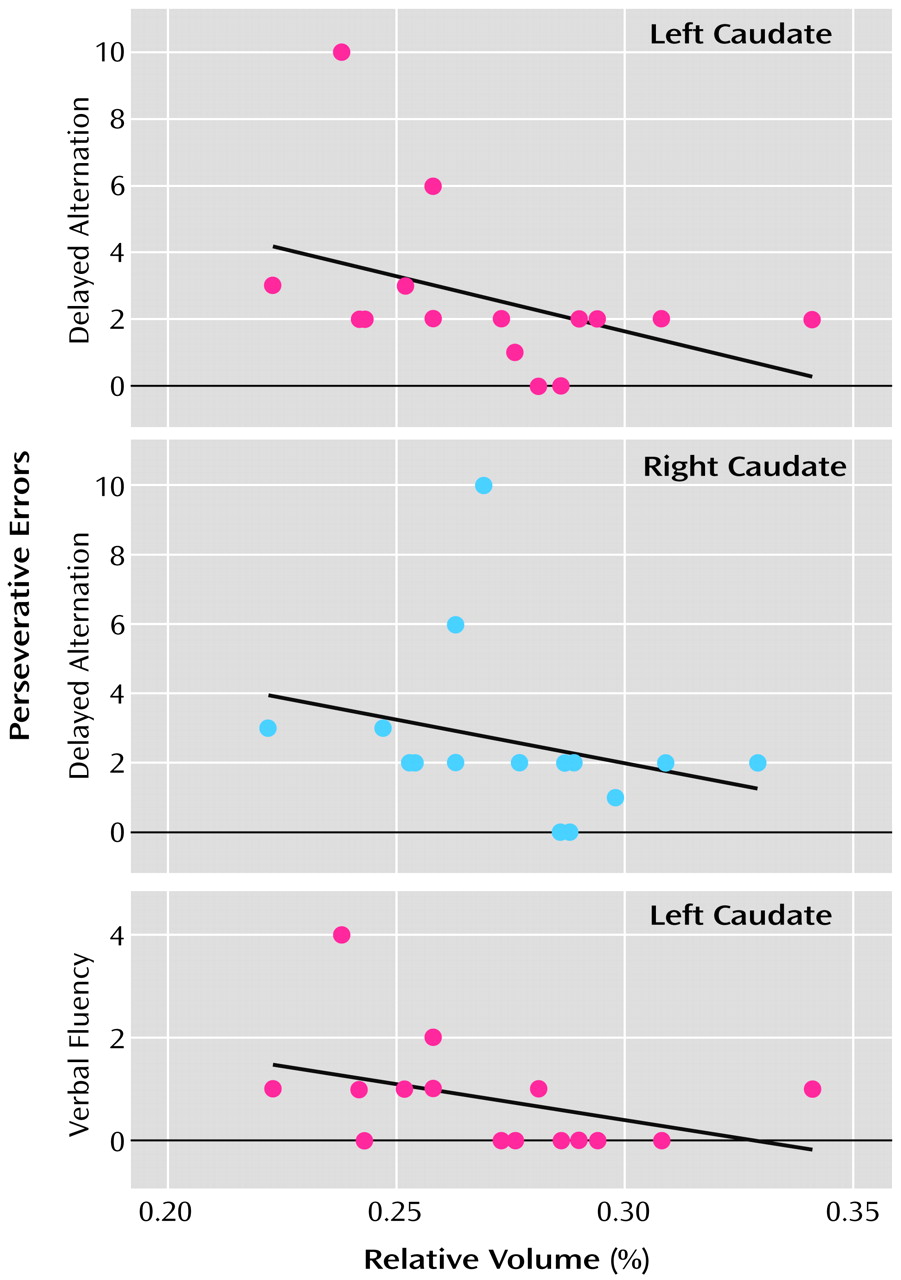
We found significant inverse correlations between caudate nucleus volumes and perseveration in both spatial and verbal working memory domains (
Figure 3). Specifically, we found bilateral negative correlations between number of perseverative errors on a delayed alternation spatial working memory task and both left (r
s=–0.55, N=15, p=0.03) and right (r
s=–0.51, N=15, p=0.05) caudate nucleus relative volume. Moreover, in a more lateralized pattern, we found a significant negative correlation between number of perseverative errors on a verbal fluency working memory task and left (r
s=–0.51, N=15, p=0.05) but not right (r
s=–0.45, N=15, p=0.09) caudate nucleus relative volume.
When we correlated the volume of the anterior, or head, of the caudate nucleus in an exploratory fashion with the measures of working memory, we found a similar, if slightly more robust, pattern of correlations. In subjects with schizotypal personality disorder, we found that the left (rs=–0.62, N=15, p=0.02) and right (rs=–0.51, N=15, p=0.05) anterior caudate relative volumes were significantly inversely correlated with number of perseverative errors on our delayed alternation working memory task. In a more lateralized fashion, the left (rs=–0.54, N=15, p<0.04) but not the right (rs=–0.20, N=15, p=0.49) anterior caudate nucleus relative volume was significantly negatively correlated with number of perseverative errors on our verbal fluency working memory task.
For clinical measures in subjects with schizotypal personality disorder, we did not find significant correlations with either overall SANS scores or with our overall measure of negative features based on a summary score of SCID-P negative feature items. Finally, we found no differences between subjects with schizotypal personality disorder and normal comparison subjects for left-hand or right-hand finger tapping (
Table 1). Furthermore, we found that finger tapping in subjects with schizotypal personality disorder did not significantly correlate with any of our caudate region of interest volumes (0.12<p<0.98).
Discussion
There were two major findings in our study not previously reported to our knowledge. First, after correcting for head size and covarying for age, we found that the relative volumes of the left and right caudate nucleus in subjects with schizotypal personality disorder were significantly smaller than those of normal comparison subjects by 9.1% and 9.2% (or 13.1% and 13.2% for absolute volumes). Furthermore, we found a bilateral inverse correlation in our subjects with schizotypal personality disorder between the volume of the caudate nucleus and the severity of perseveration in a delayed alternation spatial working memory task
(29) as well as a lateralized inverse correlation between the left caudate nucleus and the severity of perseveration on the Controlled Oral Word Association Test
(23), a verbal fluency working memory task. In exploratory analyses examining the parcellated head of the caudate nucleus, we similarly found that the left and right anterior caudate relative volumes were smaller than those of normal comparison subjects by 9.1% and 6.4% (or 13.2% and 10.1% for absolute volumes). We also found a bilateral inverse correlation between the volume of the head of the caudate nucleus and the severity of perseveration on our spatial working memory task and a lateralized inverse correlation between the volume of the left head of the caudate nucleus with the severity of perseveration in our verbal working memory task.
Our findings are important because they represent the first such findings in an unmedicated group of patients with a disorder believed to be in the schizophrenia spectrum. We found not only smaller caudate nucleus volumes in these patients but also significant correlations between structural deficits and cognitive psychopathology. It is of particular interest that we found bilateral correlations between smaller caudate nucleus volume and spatial working memory but left lateralized correlations with verbal working memory. These findings are consistent with previous conceptualizations regarding a more bilateral contribution of brain structures for spatial working memory and a more lateralized contribution of brain structures for verbal working memory
(4).
The reduction in caudate nucleus volume that we report occurred in the absence of lateral ventricular enlargement. This suggests that our findings are not simply secondary to an expansion in lateral ventricular size. Buchsbaum et al.
(34) reported on the volume of parcellated lateral ventricles in subjects with schizophrenia, subjects with schizotypal personality disorder, and normal volunteers. They found an intermediate extent of left-greater-than-right asymmetry in the temporal horn in subjects with schizotypal personality disorder than in normal subjects and subjects with schizophrenia. Certain differences between the study of Buchsbaum et al. and our study deserve emphasis. First, we measured that part of the ventricles contiguous to the caudate nucleus and, hence, did not include the temporal horn. Second, our sampling of subjects differed from that of Buchsbaum et al. in that we did not include women, our subjects were younger, and all of our subjects were right-handed. Finally, our tracing strategies delineating the ventricles differed from those of Buchsbaum et al.
A key strength of our study is that we have eliminated the confound of neuroleptic treatment, which affects the size of the basal ganglia. MRI studies of the striatum have shown that subjects who were experiencing their first episode of schizophrenia, have minimal neuroleptic exposure, and are maintained on conventional neuroleptics show enlargement in caudate nucleus volume
(35,
36), which may then be reversed by treatment with clozapine
(37). Both neural and vascular explanations have been offered for this repeated observation
(22). We believe that our study in neuroleptic-naive subjects with schizotypal personality disorder complements studies of neuroleptic-naive subjects experiencing their first episode of schizophrenia or schizoaffective disorder (e.g., references
21,
22). The convergent findings in these studies of smaller caudate nucleus volume in these two unmedicated subject groups support the view that there is an intrinsic abnormality in the caudate nucleus in the schizophrenia spectrum.
A shortcoming is that our study group is relatively small and the generalizability of these findings to all subjects with schizotypal personality disorder, a heterogeneous group, may be diminished. Nonmedicated subjects with schizotypal personality disorder who have severe problems but who, by and large, are not in treatment and have never taken neuroleptic medication are difficult to locate. Note the extensive search required (303 subjects were initially screened) to find a relatively small group of subjects who met our stringent research criteria. However, the restriction of the study group to men reduces one aspect of heterogeneity. For future studies, an additional potential approach to reduce heterogeneity would be to subtype subjects with schizotypal personality disorder into those with and without a positive family history of schizophrenia.
A further potential methodological shortcoming of our study also relates to the small number of subjects and the number of correlations with psychopathological measures that we performed. We have attempted to address this issue by basing our correlations on a priori hypotheses. The correlations we found were in the expected direction and were hypothesis driven. We had hypothesized that diminished volume in the caudate nucleus would have an impact on “frontally” mediated neuropsychological traits, such as working memory, or “frontally” mediated clinical symptoms, such as abulia or negative symptoms. In addition, our cognitive psychopathological correlations support the concept that our findings regarding the caudate nucleus are not simply epiphenomena but, instead, abnormalities related to core abnormalities in schizotypal personality disorder itself. Perhaps, because of the greater difficulty in quantitatively measuring the kind of subtle negative symptoms characteristic of our group of subjects with schizotypal personality disorder compared with measuring the number of perseverative errors in our cognitive measures, only our neuropsychological correlations are meaningful. Future studies involving larger groups of subjects are needed to confirm the findings we report here regarding the caudate nucleus in schizotypal personality disorder, particularly our findings regarding correlations between smaller caudate volume and cognitive impairment in working memory.
In sum, our finding of diminished caudate nucleus volume and associated cognitive psychopathological correlates in neuroleptic-naive subjects with schizotypal personality disorder implicates the striatum as potentially intrinsically abnormal in this disorder and other schizophrenia spectrum conditions. Our finding supports the importance of examining both cortical and subcortical regions as possible sites of pathology in this heterogeneous condition. Furthermore, it emphasizes that dysfunction in cortical-subcortical circuits may play a key role in disorders of cognition and behavior such as schizotypal personality disorder.