Hypercortisolemia is well documented in depression, and elevated cortisol levels impair learning and memory in humans
(1). The neurocognitive deficits observed in depressive disorder, therefore, may be partially attributable to glucocorticoid hypersecretion. Substantially less is known about the role of other adrenal steroids in depressive illness, particularly dehydroepiandrosterone (DHEA), which in its sulfated form is the most abundant adrenal steroid in humans. Animal studies show that DHEA counteracts the deleterious effects of corticosteroids on long-term potentiation, a neurophysiological correlate of learning and memory
(2). Improvements in cognition have been observed after administration of DHEA to middle-aged and elderly depressed patients
(3), and the antidepressant actions of DHEA have been demonstrated in a small randomized controlled trial
(4).
Since DHEA levels regulate glucocorticoid action in the brain, the ratio of cortisol to DHEA most accurately reflects the degree of “functional” hypercortisolemia. Evidence suggests that an elevated cortisol-DHEA ratio may be an additional state marker of depressive illness
(5). However, administration of antidepressants may cause changes in steroid hormones, and groups of well-defined, drug-free subjects have not been examined. Therefore, we measured basal cortisol and DHEA secretion in patients with major depression who had been medication-free for a minimum of 6 weeks and compared the results with those from healthy comparison subjects.
Method
Forty-four patients with DSM-IV-confirmed unipolar major depression were recruited from family practice clinics; five fulfilled criteria for melancholia. Twenty-nine of the patients were women, and 15 were men. All were between 18 and 55 years old (mean=33, SD=11) and had no current diagnosis of substance abuse or dependence. Twenty-six of the patients were entirely drug-naive at the time of testing. Among the 18 who had previously received psychotropic medication, the amount of time drug-free ranged from 6 to 336 weeks (median=48 weeks). Depressive symptom scores in the patient group ranged from 18 to 38 (mean=29, SD=5) on the Montgomery-Åsberg Depression Rating Scale
(6) and from 15 to 30 (mean=21, SD=4) on the 17-item Hamilton Depression Scale. The median duration of the current depressive episode was 6 months (mean=13, SD=24). Thirty patients (68%) were experiencing their first episode of depression. None had previously received ECT. As has been reported elsewhere
(7), the depressed patients displayed a range of neurocognitive deficits on tests of working memory, visuospatial recognition memory, sustained attention, verbal recall, and recognition compared with healthy subjects.
The comparison group consisted of 45 healthy volunteers. Twenty-nine were women, and 16 were men. They ranged in age from 19 to 61 years (mean=32, SD=11) and had no current medical or psychiatric illness. Subjects with personal or family history of psychiatric illness or personal history of substance misuse were excluded.
Current alcohol intake among all subjects was less than 28 units per week for men and less than 21 units for women (unit=10 ml of alcohol). The groups were well matched for age, sex, season of testing, and, for women, phase of menstrual cycle. After a complete description of the study, written informed consent was obtained from all participants; the study received full approval from the local ethics committee.
Saliva samples were collected at 8:00 a.m. and 8:00 p.m. over 2 consecutive days; citric acid-treated cotton salivettes (Sarstedt, Leicester, U.K.) were used. An average for each time point was then calculated. Five patients and four comparison subjects delivered incomplete samples and were excluded from the analysis. Data were available for 39 patients and 41 comparison subjects. Cortisol was determined by using Corti-cote radioimmunoassay kits (ICN Pharmaceuticals, Costa Mesa, Calif.), and DHEA was determined by validated radioimmunoassay (Bioclin, Cardiff, U.K.) following sample extraction into hexane/ether (4:1). The interassay coefficient of variation (low concentration=70 pg/100 μl) for cortisol was less than 10%, and the intraassay variation was less than 7.3%. For DHEA, both the interassay coefficient of variation and the intraassay variation were less than 6.5%.
The data were analyzed by repeated measures analysis of variance (ANOVA) (SPSS 9.0, SPSS, Inc., Chicago) with diagnosis (depressed or healthy) and sex as between-subject factors and time of sample (8:00 a.m. or 8:00 p.m.) as a within-subject factor. Logarithmic transformations (base-10) were applied to the data to normalize the distribution.
Results
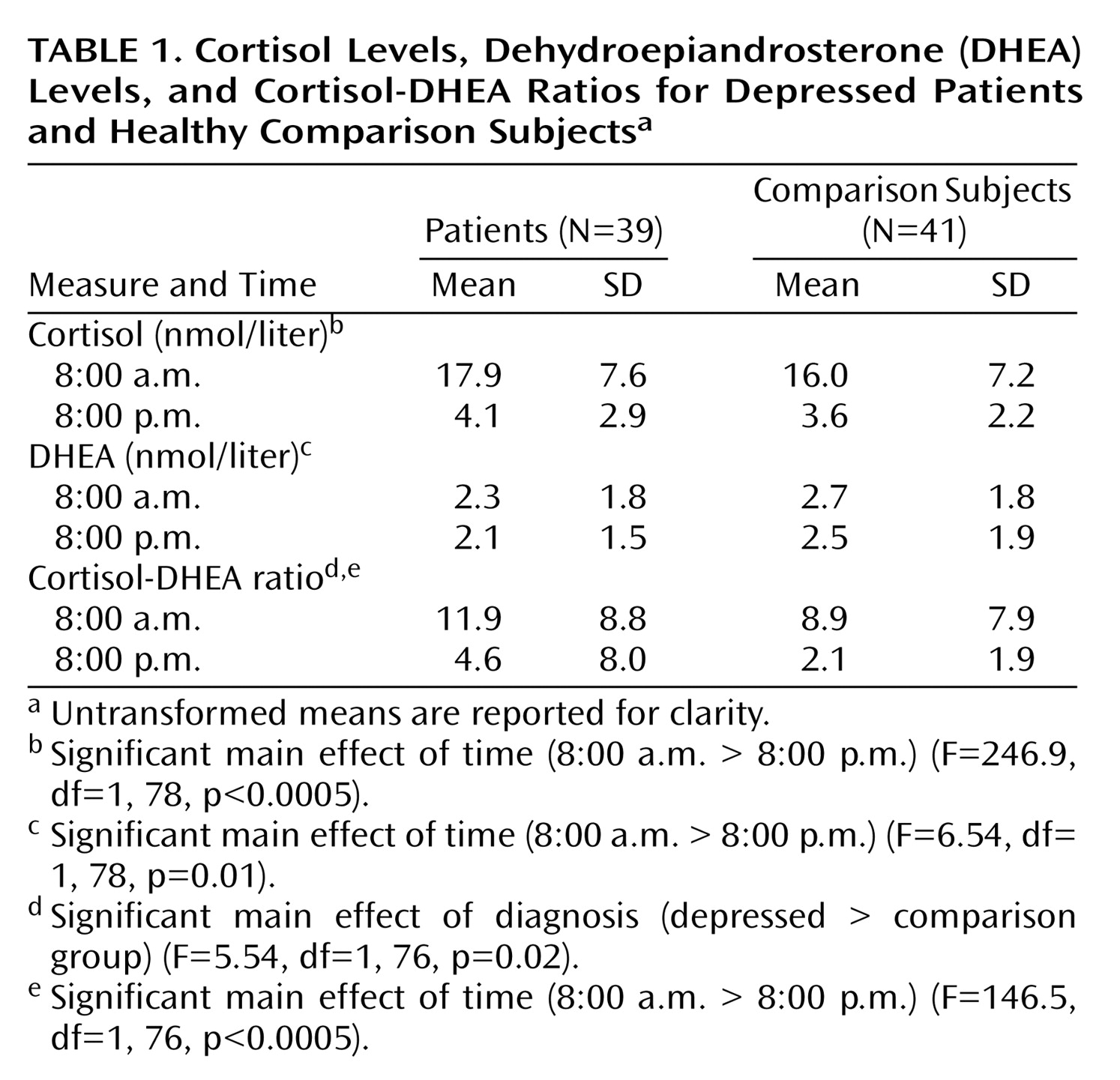
The molar cortisol-DHEA ratio was significantly greater in depressed patients (log-10-transformed mean=0.67, SD=0.37) than comparison subjects (log-10-transformed mean=0.49, SD=0.32) (
Table 1) with no significant main effect of sex (F=0.22, df=1, 76, p=0.64) and no sex-by-diagnosis interaction (F=3.14, df=1, 76, p=0.08). When analyzed separately, cortisol (F=3.59, df=1, 78, p=0.06) and DHEA (F=3.57, df=1, 78, p=0.06) levels did not significantly differ. Again, there was no main effect of sex and no sex-by-diagnosis interactions. There was no significant time of sample-by-diagnosis interaction (F=0.35, df=1, 76, p=0.56). The cortisol-DHEA ratio and, separately, cortisol and DHEA exhibited significant diurnal variation; levels were lower at 8:00 p.m. (
Table 1).
Age did not correlate with cortisol, DHEA, or cortisol-DHEA ratio at any time point. In depressed patients, the 8:00 p.m. cortisol-DHEA ratio correlated positively with length of current depressive episode (rs=0.35, N=40, p<0.03).
Discussion
In this study, the cortisol-DHEA ratio was higher in depressed patients than healthy comparison subjects, adding further to the evidence of a “functional” hypercortisolemia in depression. The lack of hypercortisolemia in the depressed group is not surprising because low rates of hypercortisolemia are typically reported in nonmelancholic outpatients. Because previous studies of DHEA in depression have suggested that both antidepressant drugs and age may be confounding factors, we recruited patients who had been drug-free for a minimum of 6 weeks and who were considerably younger than those participating in earlier studies
(5).
Plasma levels of cortisol and DHEA can be measured precisely; however, the stress of venepuncture may elevate steroid levels. Saliva sampling is noninvasive and easier to perform with subjects whose hypothalamic-pituitary-adrenal axis responses are potentially more sensitive to stressful interventions. Alternative methods of saliva sampling have been suggested
(8) that may be more accurate than those used in this study. Verification of our preliminary findings by studies using these collection methods is warranted. Self-reporting of drug abuse may be unreliable, and future studies should also perform routine urine drug screening.
Prolonged exposure to excess glucocorticoids leads to neuronal atrophy
(9). Another study with the same drug-free, predominantly nonmelancholic depressed patients assessed in the current study
(7) found widespread neurocognitive impairments, which may be a direct consequence of the patients’ elevated cortisol-DHEA ratio. Administration of DHEA or other antiglucocorticoids may reduce their neurocognitive impairment.
DHEA has effects on several receptor systems. In addition to antiglucocorticoid actions, these include sigma
1-receptor agonism and GABA
A receptor antagonism. Neurosteroids with these pharmacological properties offer a novel therapeutic approach in mood disorders
(4,
10).