Major depressive disorder afflicts an estimated 17.1% of individuals during their lifetimes
(1). Major depressive disorder is a major health problem associated with gradual and often incomplete recovery
(2,
3), significant limitations in functioning and well-being
(4–
6), greater risk of mortality due to medical illness
(7), increased utilization of health services
(7), and tremendous costs to society
(8).
Fortunately, there are a number of efficacious medications and/or psychotherapies from which clinicians may choose when treating a depressed patient
(9,
10). Several meta-analyses comparing the efficacy of these treatments have been published
(10–
13). However, these meta-analyses have several methodological limitations. First, several
(11–
13) included studies that did not require the use of standardized diagnostic criteria for major depressive disorder and studies of less severely “depressed” student groups. Second, some
(12,
13) did not separate treatment effects on the basis of the type of analysis performed (completer versus intent-to-treat). Third, no attempt was made in any of the meta-analyses to ensure that the treatments were “validly compared, within assay-sensitive randomized studies,” as recently suggested by Klein
(14), which raises concerns about the validity of the comparisons. Finally, efficacy was measured in terms of effect size
(11–
13) or response to treatment
(10), usually defined as a reduction of at least 50% in the score on a depression rating scale. A limitation of this approach is that some “responders” may still have a substantial degree of residual depressive symptoms, which increases the risk of relapse, social and vocational impairment, and suicide
(6,
15–19). Therefore, it may be more clinically meaningful to measure efficacy in terms of full remission, defined as “a relatively brief…period during which…the individual is asymptomatic (i.e., no longer meets syndromal criteria for the disorder and has no more than minimal symptoms)”
(20).
In this review we examine the comparative efficacy of pharmacotherapy and psychotherapy for major depressive disorder. Although several similar meta-analyses have already been published, we aim to overcome the limitations of previous meta-analyses by including only multiple-cell randomized, controlled trials that directly compared pharmacotherapy, psychotherapy, and control conditions for patients with well-defined major depressive disorder and by using full remission as the main dependent variable. To our knowledge, ours is the first review to use such methods, making it a useful addition to the meta-analytic literature on the topic.
Method
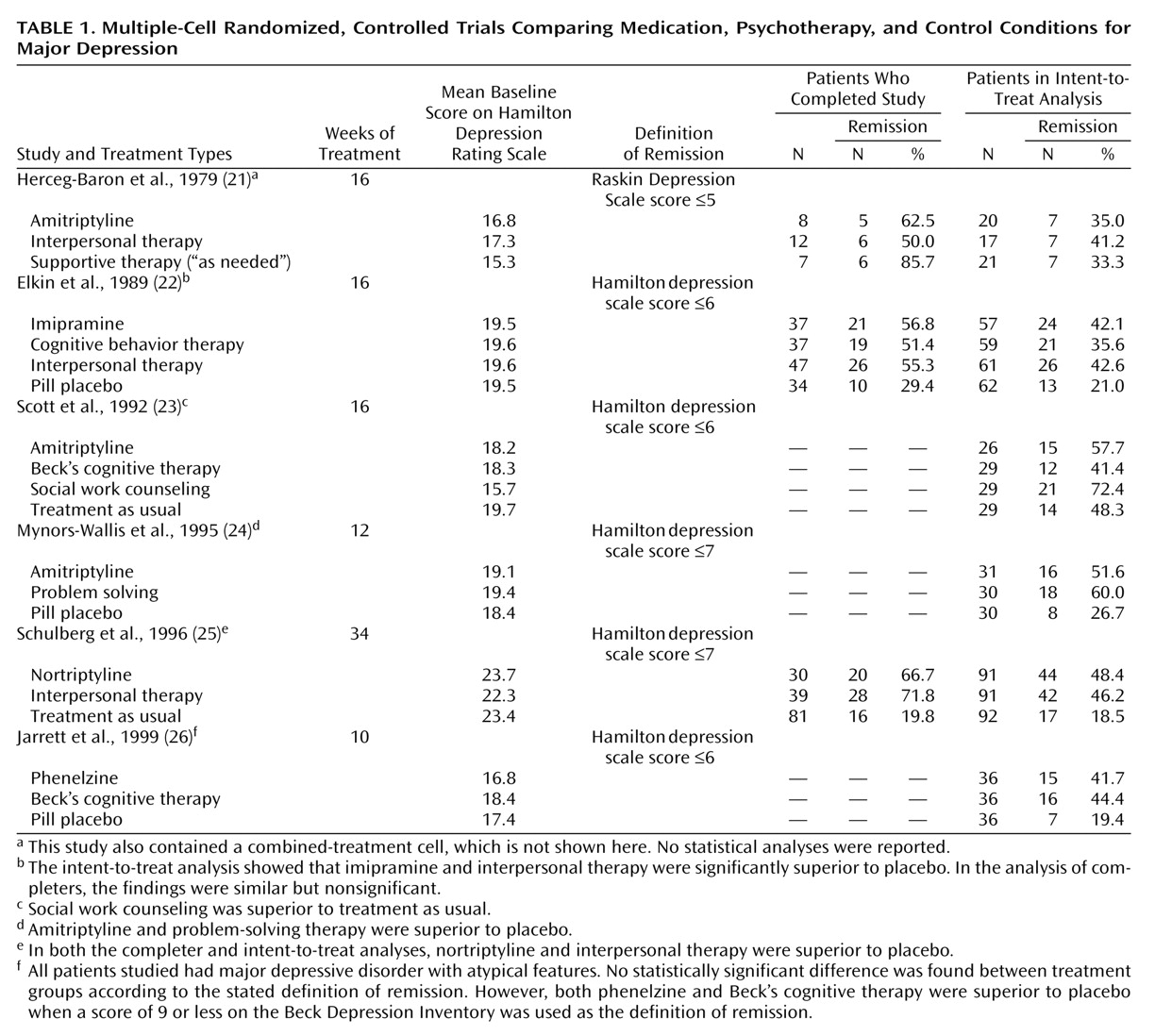
Treatment studies of major depressive disorder were identified by using computerized searches of the MEDLINE and PsychINFO databases up to November 2000. The key terms “depressive disorder” and “major depression” were used with their subheadings “drug therapy”/“therapy” and “treatment and prevention,” respectively, for MEDLINE and PsychINFO. This process was complemented by a manual search of references cited in retrieved articles and pertinent reviews on the topic. The included studies 1) were published in English-language journals, 2) focused on adults, 3) used standardized diagnostic criteria for major depressive disorder, 4) used a multiple-cell randomized, controlled trial design, and 5) were reported with sufficient data to allow the calculation of remission percentages. Published articles by the same research group that focused on the same study group were pooled to ensure the independence of studies included in our review. Our inclusion criteria were met by six main articles
(21–
26) presenting efficacy data and seven companion articles
(27–
33) with additional relevant data.
Remission was calculated by using both completers and intent-to-treat groups, as available. However, it should be noted that in one study
(25) all patients in the treatment-as-usual control group were considered completers unless they refused to be contacted for follow-up. Furthermore, whereas for most studies the intent-to-treat data were based on a last-observation-carried-forward analysis of all patients randomly assigned to treatment, one study
(21) examined the remission status of all patients at the end of the study, including dropouts who had received further nonrandomized treatment, while in another
(23) all dropouts were considered to be “not recovered.”
Data analysis was performed by using SAS, version 8.0 (Cary, N.C., SAS Institute). Categorical variables were tested by using the chi-square statistic. Continuous variables were examined by using general linear model procedures followed by Duncan’s multiple range test for multiple comparisons. All significance tests were performed at a two-tailed alpha level of 0.05.
Discussion
The novel contributions of our review are twofold. First, our focus was on remission instead of effect size
(11–
13) or response
(10). The finding that there were significantly more patients in the Elkin et al. study
(22) who responded to treatment than achieved full remission lends support to the hypothesis that some “responders” may still have a substantial degree of residual depressive symptoms, thereby confirming that the focus on full remission represents an important paradigm shift in the field. Second, to our knowledge, this is the first review that examines the comparative efficacy of pharmacotherapy and psychotherapy for major depressive disorder exclusively on the basis of the results of multiple-cell randomized, controlled trials that directly compared medications, psychotherapies, and control conditions. This approach, as suggested by others
(14), is the only safeguard against noncomparability of study groups, inadequate implementation of pharmacotherapy, and nonspecific treatment effects. We also examined a more homogenously defined patient group than was covered in other meta-analyses
(11–
13), including only studies of adults meeting standardized diagnostic criteria for major depressive disorder.
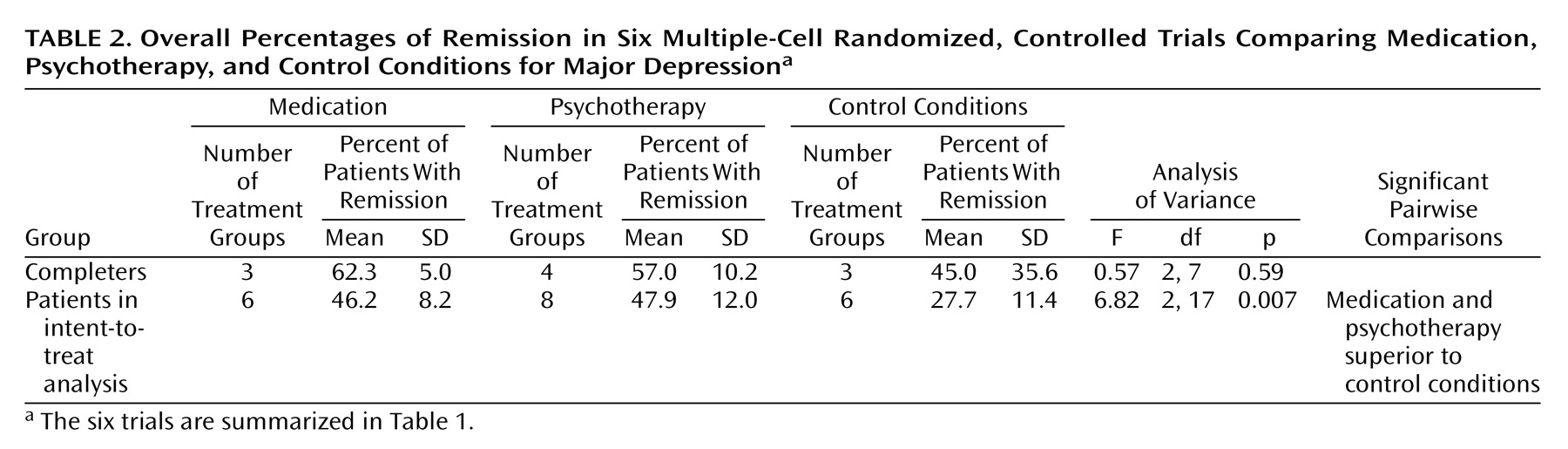
Based on this approach, the main finding of this review is that both antidepressant medication (tricyclic antidepressants and phenelzine) and psychotherapy (primarily cognitive behavior therapy and interpersonal therapy) are almost twice as efficacious as control conditions in producing full remission in outpatients with primarily nonmelancholic nonpsychotic major depressive disorder of mild to moderate severity after a median of 16 weeks of treatment; i.e., intent-to-treat analysis indicated that the mean percentages of patients with remissions after active treatments were approximately 46% for medication and psychotherapy, compared to 24% for control conditions. The lower attrition percentages in the groups receiving active treatments than in those in control conditions may also reflect the greater efficacy of active treatments, although this remains speculative in the absence of adequate reporting of reasons for dropout/withdrawal for the original studies. Although our results contrast with those of Dobson
(11), who concluded that cognitive therapy was more efficacious than pharmacotherapy for “depressed” patients, more recent meta-analyses
(10,
12,
13) failed to demonstrate a significant difference in efficacy between psychotherapy and medication, particularly once the effect of investigator allegiance was accounted for
(12,
13). The finding that the percentage of remission produced by psychotherapy is similar to the percentage for medication is relevant for clinicians when treating mildly to moderately depressed outpatients with relative contraindications to medication, such as pregnancy
(36) or intolerable adverse effects, and in view of reports of greater acceptability of psychotherapy for depression
(37,
38).
The results of our review must be interpreted in the light of several potential limitations. First, the subjects in the original studies were primarily adult outpatients with unipolar, nonpsychotic major depressive disorder of mild to moderate severity. Therefore, the generalizability of our results is uncertain for child, adolescent, and elderly patients, patients with treatment-resistant, bipolar, or psychotic depression, and those with significant substance use disorders or general medical conditions, all of whom were excluded from the original randomized, controlled trials. Second, the results may or may not be generalizable to the newer antidepressants, which have largely replaced tricyclic antidepressants as first-line medications for major depressive disorder. Furthermore, treatments delivered in controlled trials are rarely carried out under routine clinical conditions
(39). For example, whereas an ineffective antidepressant would be continued for 10–16 weeks in controlled trials, two antidepressant trials could be performed in a similar time frame in routine clinical practice. These factors may have led to an underestimation of the efficacy of antidepressant medication in the original multiple-cell randomized, controlled trials, a possibility that is supported by the results of a recent open-label study
(40). Similarly, in clinical practice, when a patient fails to improve with a manualized psychotherapy, a good psychotherapist might shift to a different therapeutic approach. Hence, these studies may underestimate the efficacy of psychotherapy as well. Regarding treatment setting, three studies were performed in primary care
(23–
25), while three others
(21,
22,
26) were carried out in psychiatric settings. Although the likelihood of achieving remission was found to be significantly greater for patients in primary care than in psychiatric settings, no interaction was found between treatment setting and treatment type.
Third, the reports on the original studies often failed to include data on illness characteristics that may affect the interpretation of results, such as comorbidity and the percentage of patients with chronic, atypical, or melancholic depression. For example, given that approximately 28%–42% of depressed clinic patients have atypical features
(41–
43) and that imipramine has been shown to be less efficacious than cognitive behavior therapy for atypical depression
(35), it is possible that our results underestimate the comparative efficacy of antidepressant medications relative to psychotherapy.
Fourth, identification of possible predictors of remission was limited by the low statistical power related to the small number of available multiple-cell randomized, controlled trials, as well as the lack of variance for such variables as severity of depression and treatment duration. Regarding severity, there are conflicting reports in the literature as to whether initial depression severity predicts a differential treatment effect
(44,
45) or whether greater baseline severity of depression predicts poorer outcomes regardless of treatment type
(46). Regarding treatment duration, the percentage of patients with major depressive disorder who achieved recovery each month in one naturalistic study
(2,
47) was 11%–15% during the first 3 months of follow-up before dropping to 3%–5% for the next 9 months. Exclusive use of multiple-cell randomized, controlled trials did not allow us to adequately address severity of depression or time required to attain optimal remission percentages with either active treatment.
Fifth, all studies in this review focused on remission at one point in time but did not require it to be sustained for any duration. As a result, the focus of this review is on full remission, not recovery. Ideally, treatment studies of major depressive disorder should examine both remission and recovery.
Sixth, although publication bias is a concern in systematic reviews, we regard the existence of unpublished multiple-cell randomized, controlled trials for major depressive disorder as highly unlikely given the relevance, cost, and high visibility of such studies. Furthermore, statistical methods to detect publication bias were not applicable to this review since it lacks a sufficient number of individual studies with a range of group sizes
(48).
Last, our review does not address other important issues, such as the relative cost, speed of onset of therapeutic effect, availability, and prophylactic efficacy of treatments or possible differential treatment effects on measures of functioning or core psychopathology. It also does not examine the comparative efficacy of combined pharmacological and psychological treatments. These and other issues already noted warrant further attention in a wider range of studies.
On the basis of a review of all available multiple-cell randomized, controlled trials directly comparing the efficacy of pharmacotherapy, psychotherapy, and control conditions for patients with well-defined major depressive disorder, we found that both active treatments produced higher percentages of patients with remissions and lower attrition percentages than control conditions but that these percentages were not significantly different from each other. Therefore, we conclude that both psychotherapy and pharmacotherapy may be considered first-line treatments for mild to moderately depressed outpatients seen in general medical practice or psychiatric treatment settings.