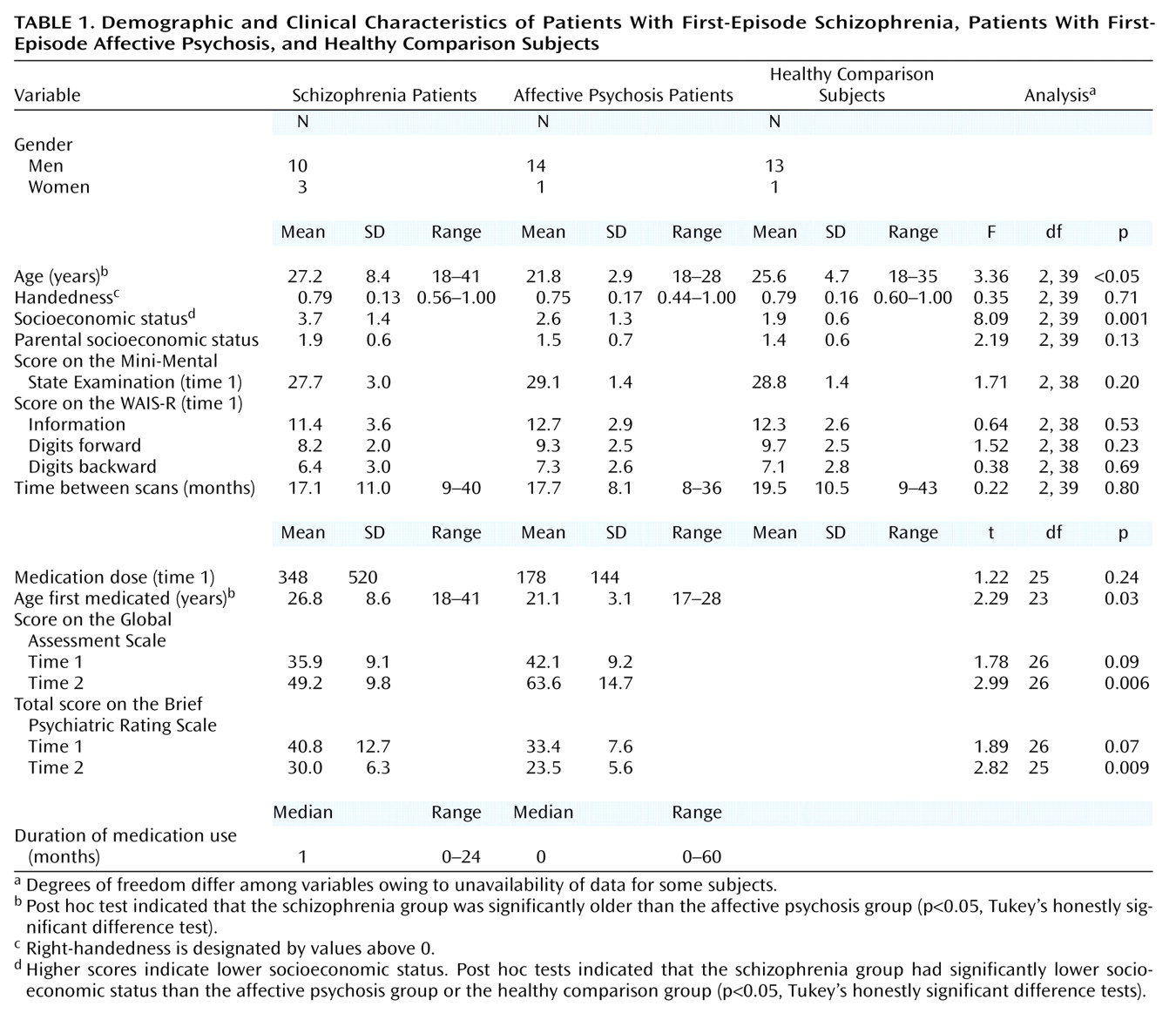
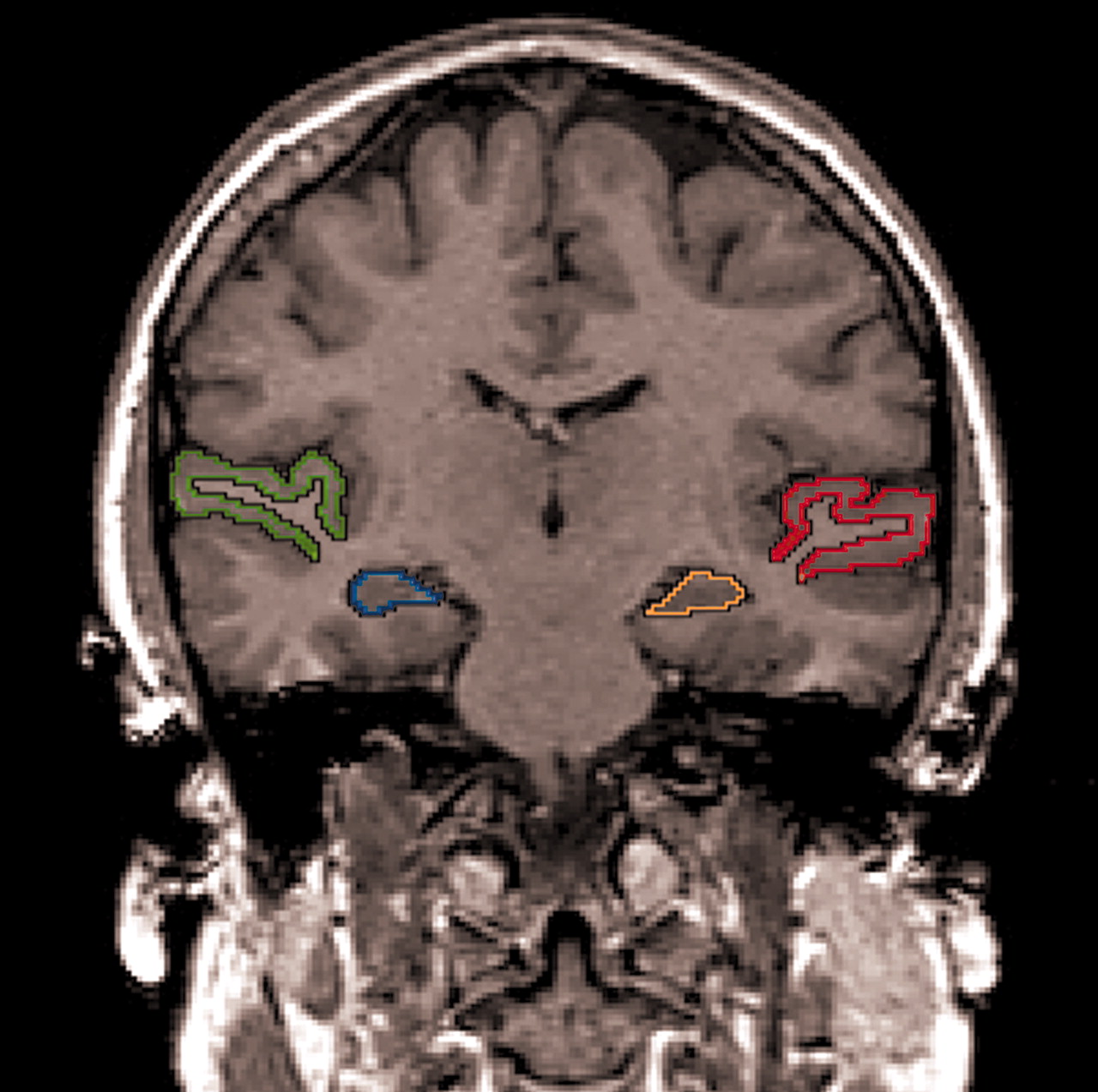
Moreover, several investigators have also reported a volume reduction of temporal lobe gray matter regions of interest in patients with first-episode schizophrenia, including the superior temporal gyrus
(5,
6) and hippocampus
(6,
7). Our laboratory noted smaller left posterior superior temporal gyrus
(8) and smaller left planum temporale and total (left plus right) Heschl’s gyri gray matter volumes
(9) in patients with first-episode schizophrenia that were not present in patients with first-episode affective (mainly manic) psychosis. On the other hand, both groups showed smaller left-side posterior amygdala-hippocampal complex volumes
(8). This different pattern of gray matter reductions supports the hypothesis that schizophrenia and affective psychoses are different disorders, although it does not rule out the possibility of epistatic or environmentally induced variations of the same genetic etiology.
However, only longitudinal studies can provide definitive evidence about progression. Ideally, longitudinal studies should 1) use high-spatial-resolution MRI technology (currently 1.5-mm contiguous slices), 2) examine several regions of interest, 3) segment gray and white matter (this procedure provides the most accurate measure [
3–
4]), 4) start the longitudinal study group at the first episode, and 5) demonstrate specificity to schizophrenia vis-à-vis another functional psychosis with a frequently chronic course, such as manic or unipolar psychosis.
A number of investigators have pioneered longitudinal MRI studies of temporal lobe structures in schizophrenia patients
(5,
15–19), although the findings have been somewhat controversial. Mathalon et al.
(19) demonstrated that male patients with chronic schizophrenia exhibited more volume decline than healthy comparison subjects in bilateral posterior superior temporal gyrus gray matter. Additionally, Jacobsen et al.
(17) reported that patients with childhood-onset schizophrenia showed significantly greater decreases than healthy comparison subjects in the right temporal lobe, superior temporal gyrus, and left hippocampal volumes. Other studies of patients with first-episode schizophrenia, however, indicated no faster decreases than those of healthy comparison subjects in the hippocampus
(15,
18) or (unsegmented) temporal lobes
(15,
16). Keshavan et al.
(5) reported a reversal of volume reduction in the initial gray matter superior temporal gyrus with neuroleptic treatment after 1 year of follow-up in patients with first-episode schizophrenia. However, to our knowledge, no study to date has evaluated progressive changes in superior temporal gyrus and amygdala-hippocampal complex gray matter volumes in patients with first-episode schizophrenia as contrasted with those in patients with first-episode affective psychosis.
We here report longitudinal data indicating a progressive reduction in gray matter volume of the left posterior superior temporal gyrus in patients with schizophrenia compared with those of patients with affective psychosis and healthy comparison subjects.
Results
Volume Changes
Age, socioeconomic status, parental socioeconomic status, age first medicated, duration of medication treatment, medication dose, and intracranial contents volume at time 1 or time 2 did not correlate with any of the region-of-interest volumes at time 1 or time 2 or with the change in any of the region-of-interest volumes in either patient group.
Although Shapilo-Wilk tests indicated that percent changes in the left and right amygdala-hippocampal complex in schizophrenia patients were not normally distributed (W=0.86, df=13, p<0.05; W=0.84, df=13, p=0.002, respectively), there was no violation of sphericity for the repeated measures ANOVA as evaluated by Mauchly’s test for sphericity (Mauchly’s W=1.0). For confirmatory purposes, we also used arcsine transformations, which did not alter the statistical results described.
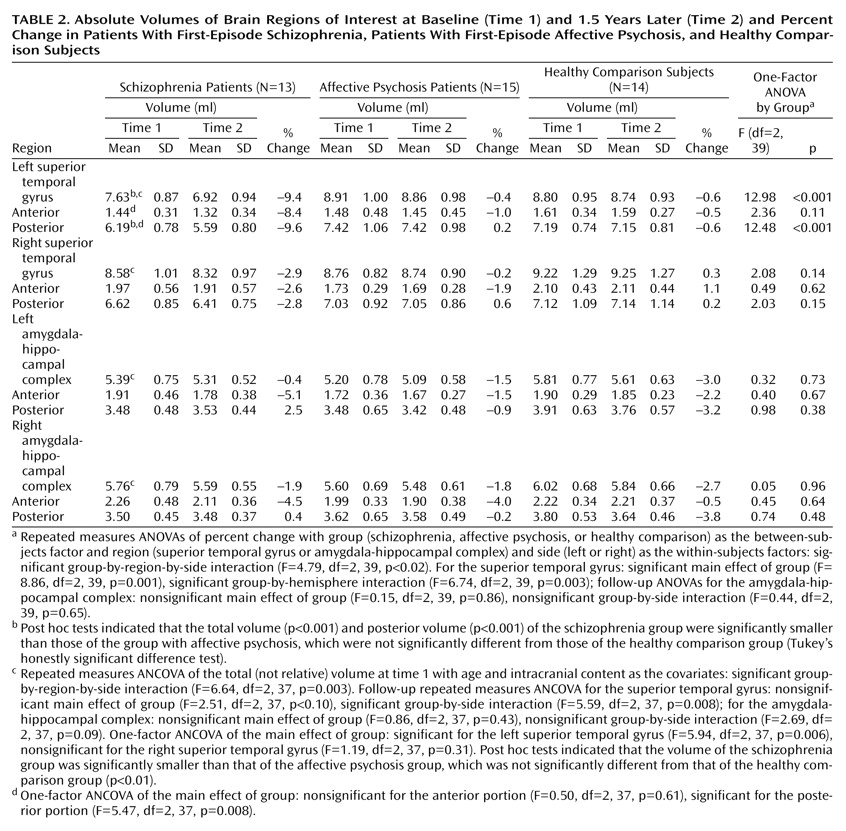
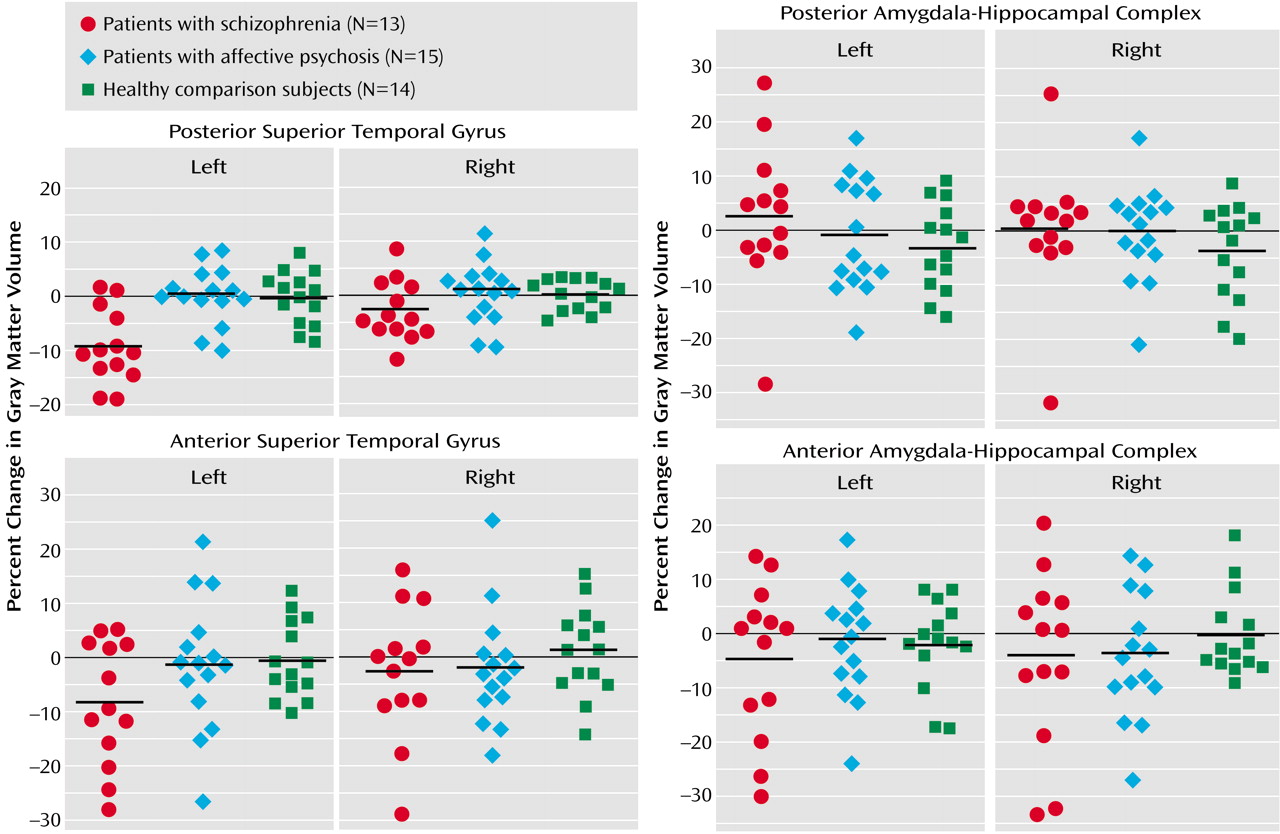
Groups were not significantly different in overall change in volume across regions (F=0.86, df=2, 39, p=0.43) (
Table 2,
Figure 2). However, a significant group-by-region interaction (F=4.79, df=2, 39, p<0.02) and a significant group-by-region-by-side interaction (F=4.79, df=2, 39, p<0.02) indicated that a group difference was present in at least one region. Thus, analyses were conducted separately on the superior temporal gyrus and amygdala-hippocampal complex. Groups did not differ significantly regarding the amygdala-hippocampal complex (F=0.15, df=2, 39, p=0.86), and there was no group-by-side interaction (F=0.44, df=2, 39, p=0.65).
In marked contrast, groups were significantly different regarding the superior temporal gyrus (F=8.86, df=2, 39, p=0.001), and the degree of group difference was significantly different between hemispheres (F=6.74, df=2, 39, p=0.003). We next compared the left and right superior temporal gyrus separately. Groups were significantly different regarding the left superior temporal gyrus (F=12.98, df=2, 39, p<0.001), with schizophrenia patients showing a significantly faster volume decrease than both affective psychosis patients and healthy comparison subjects (p<0.001, Tukey’s honestly significant difference test). On the other hand, groups were not different regarding the right superior temporal gyrus (F=2.08, df=2, 39, p=0.14).
To further isolate the locus of change in the superior temporal gyrus, we next performed group comparisons separately for the anterior and posterior portions of the left superior temporal gyrus using the one-factor ANOVA. Significant group differences were observed for the left posterior superior temporal gyrus (F=12.48, df=2, 39, p<0.001), with schizophrenia patients showing more change (9.6% decrease) than both affective psychosis patients and healthy comparison subjects (p<0.005, Tukey’s honestly significant difference). Group differences did not reach significance for the left anterior superior temporal gyrus (F=2.36, df=2, 39, p=0.11) (8.4% decrease for schizophrenia patients).
Wilcoxon’s signed rank nonparametric test revealed that only the left superior temporal gyrus in the schizophrenia group showed a significant volume change (z=–2.90, df=12, p=0.004; 11 of 13 patients showed decreases). Moreover, separate anterior and posterior superior temporal gyrus subdivision analyses showed that the schizophrenia patients had a significant change (decrease) in the left posterior superior temporal gyrus (z=–2.97, df=12, p=0.003; 11 of 13 had decreases) but that change in the left anterior superior temporal gyrus only approached significance (z=–1.89, df=12, p=0.06; 8 of 13 had a decrease). In contrast, eight of 15 affective psychosis subjects (z=–0.23, df=14, p=0.82) and seven of 14 healthy comparison subjects (z=–0.53, df=13, p=0.59) showed decreases in volume in the left superior temporal gyrus. For the remaining regions of interest, there were no significant changes for any of the groups (z=–1.85 to –0.23, df=12–14, p=0.06–0.86).
Correlations Between Volumes and Clinical Measures
For the patient groups, region-of-interest volume changes over time were not significantly associated with initial, average, or changes on the total or factor BPRS, the MMSE, the subscales of the WAIS-R, or the GAS. Of note, the left posterior superior temporal gyrus change did not significantly correlate with the interscan interval in the schizophrenia group (rs=0.285, N=13, p=0.35), driven by the fact that six of the eight patients who showed more than 10% decreases had a short interscan interval (9–11 months). In contrast, in the other groups, the left posterior superior temporal gyrus change was negatively correlated with interscan interval (the longer the interscan interval, the more change) in both the affective psychosis group (rs=–0.576, N=15, p<0.03) and the healthy comparison group (rs=–0.585, N=14, p<0.03), and both of these correlations were significantly different from that of the schizophrenia group (p<0.04, Fisher’s z transformation).
Discussion
To our knowledge, this is the first study to demonstrate a progressive reduction in left superior temporal gyrus gray matter volume in schizophrenia patients in the first 1.5 years after their first hospitalization. These results support the presence of a regionally selective progressive process in the pathophysiology of schizophrenia that is specific to schizophrenia patients as contrasted with patients with affective psychosis. Although posterior superior temporal gyrus—and not anterior superior temporal gyrus—change attained significance, we note it is possible that a larger study group might show anterior superior temporal gyrus significance. A conservative summary is that the change is selective to the left superior temporal gyrus relative to the right and is likely more pronounced in the posterior portions of the left superior temporal gyrus.
The present findings of superior temporal gyrus region-of-interest volumes at time 1 are in accordance with our earlier study
(8), replicating the specificity of smaller left posterior superior temporal gyrus volume in schizophrenia (for results, see captions to
Table 2) and showing similar results for the left posterior amygdala-hippocampal complex, although they did not reach significance. While some studies of temporal lobe morphometry in schizophrenia have not shown a left-lateralized reduction
(6), in the present group of first-episode schizophrenia patients, the left posterior superior temporal gyrus not only showed a smaller volume at time 1 but also showed a 9.6% further volume reduction over 1.5 years that was also specific to schizophrenia psychosis. It is possible that the left anterior superior temporal gyrus might also show a statistically significant progressive decrease with a larger study group size or that the left anterior superior temporal gyrus might show a somewhat delayed or slower progression than the left posterior superior temporal gyrus. These possibilities could be tested in future studies with a larger group and a follow-up over a longer period of time.
The present study’s rate of 9.6% for progressive change in the left posterior superior temporal gyrus in schizophrenia patients is about twice the rate of change of about 3% per year found in chronic schizophrenia patients in the study by Mathalon et al.
(19). Moreover, this large volume decrease appeared especially prominent in the first year, and the time course was significantly different compared with the affective psychosis and healthy comparison groups in which small and statistically nonsignificant volume reductions linearly increased with time. These data are compatible with a progressive process that is especially severe during the early stage of schizophrenia, while the affective psychosis and healthy comparison groups showed time-related changes in volume that might be expected in normal aging. Such a curvilinear decay of volume during the first year of overt psychosis that asymptotes within a year or two would be consistent with data showing less change over time in chronic schizophrenia
(19) and also consistent with the presence of an active phase of cortical deterioration early in the disease. This hypothesis of a more severe volume reduction early in the illness, however, can only be definitively tested through further repeated scans of the present cohort and of additional subjects.
The precise neurobiological mechanism underlying this progressive, perhaps neurodegenerative, change in the left posterior superior temporal gyrus is unknown. However, there is a growing body of work implicating abnormal excitatory amino acid neurotransmission in schizophrenia, possibly mediated through a deficit in recurrent inhibition
(23–
25). Recent in vivo MRI spectroscopy findings have also suggested excitatory amino acid abnormalities in schizophrenia patients
(26,
27). Although controversial, this mechanism could be a possible cause of ongoing, use-dependent cellular damage through excitotoxic effects, a mechanism that would strongly support the use of neuroleptic treatment to suppress overexcitation as well as psychosocial intervention. Alternately, there may be an abnormality in the normal synaptic pruning mechanism of late adolescence whereby schizophrenia patients overly reduce their dendritic arborization
(28), reflected in gray matter volume reductions.
Previous findings from follow-up MRI studies evaluating temporal lobe structures in patients with first-episode schizophrenia have been controversial. For example, DeLisi et al.
(15), using 5-mm-thick MRI slices with 2-mm gaps between slices, reported no volume changes over time in amygdala-hippocampal complex or temporal lobe volume. In contrast, Gur et al.
(16), using 5-mm MRI slices with no gap, reported temporal lobe volume changes in both schizophrenia patients and healthy comparison subjects after a 2–3-year follow-up period. However, neither of these studies segmented gray and white matter separately, and thus a direct comparison with our study is not possible. Keshavan et al.
(5), using 2.6-mm axial MRI slices with no gap, reported a reversal (9.0% increase) of superior temporal gyrus gray matter volume reduction with neuroleptic treatment after 1 year of follow-up in first-episode schizophrenia patients, while their healthy comparison subjects also had an increase of 6.9%. Although a paired t test comparing time 1 and time 2 scans was significant only in the schizophrenia group, no group-by-time ANOVA interaction was reported, and thus the possibility cannot be ruled out regarding unknown factors leading to a general increase in measured superior temporal gyrus volume in both healthy comparison and schizophrenia subjects in their study.
In this study, clinical measures were not significantly correlated with the percent change of the any of the region-of-interest volumes in either patient group. Mathalon et al.
(19) reported that temporal gray matter volume decline was related to greater BPRS total and negative symptom scores in their group of chronic schizophrenia patients after a mean interscan interval of 4 years. The mean 1.5-year interscan interval in our study may not be long enough for symptom correlations to emerge, as the symptom profile of patients early in the disorder may be in flux. Further studies will be also necessary to investigate the relationship between morphological changes and auditory, language, and memory function involving temporal lobe structures.
In a follow-up MRI study of schizophrenia, the use of medicated patients inevitably raises the question of whether progressive effects are possibly due to medication or to the illness itself. While the present subject group was small, the available data may be useful to present. The percent change of left posterior superior temporal gyrus volume between schizophrenia patients who received neuroleptics between scans (N=10, change, mean=–9.3%) and those who did not (N=3, mean=–10.6%) was not statistically different (Mann-Whitney U=12.00, df=11, p=0.61). We caution that (obviously) the groups were very small and that, in this naturalistic study, it was not possible to control prescan or interscan medication type or dose or to monitor medication compliance other than through hospital records and patient accounts. Since only one subject was taking typical neuroleptics between scans, comparison with subjects taking atypical neuroleptics was not possible.
However, our use of neuroleptic-medicated patients with first-episode affective psychosis may help in disambiguating whether the progressive volume decrease in schizophrenia may be at least partly independent of medication effects. In fact, the affective psychosis patients who received neuroleptics between scans (N=6, change: mean=1.7%) and those who did not (N=8, mean=–0.9%) did not differ from the healthy comparison subjects in percent change of the left posterior superior temporal gyrus volume (affective psychosis subjects taking neuroleptics versus healthy comparison subjects: Mann-Whitney U=30.00, df=18, p=0.32: affective psychosis subjects not taking neuroleptics versus healthy comparison subjects: Mann-Whitney U=52.00, df=20, p=0.79). Moreover, these two subgroups of patients with affective psychosis were not different from each other in percent change regarding the left posterior superior temporal gyrus volume (Mann-Whitney U=14.00, df=12, p=0.20). This argues against a single determinative effect of neuroleptics on gray matter volume. While mood stabilizers, especially lithium, are another potential source of medication effects
(29), exclusion of the eight patients receiving lithium between scans did not alter the statistical conclusion reported here.
Regarding the dropout rate, out of the 51 subjects reported in our earlier study
(8), more than half (N=26) had rescans used in the present study, and of importance, the dropout rate did not differ significantly among groups. Nor was the dropout rate significantly different among subjects above or below the median value of the left posterior superior temporal gyrus volume in the Hirayasu et al. study
(8) for either the schizophrenia, affective psychosis, or healthy comparison groups (p=1.00, p=0.62, p=0.34, respectively, Fisher’s exact test). Additionally, we note that the region-of-interest volume profile of the subjects in the earlier study was almost exactly replicated in the present study, further suggesting the absence of selection bias in the subjects receiving two scans.
In conclusion, the left posterior superior temporal gyrus gray matter volume reduction over time in our group of subjects with first-episode schizophrenia—but not in the group with first-episode affective psychosis—suggests that a progressive process in this specific brain region and in the early stage of the illness may play a crucial role in the pathophysiology of schizophrenia.