The Ventral Striatal-Pallidal System and Its Cortical-Subcortical Reentrant Circuits
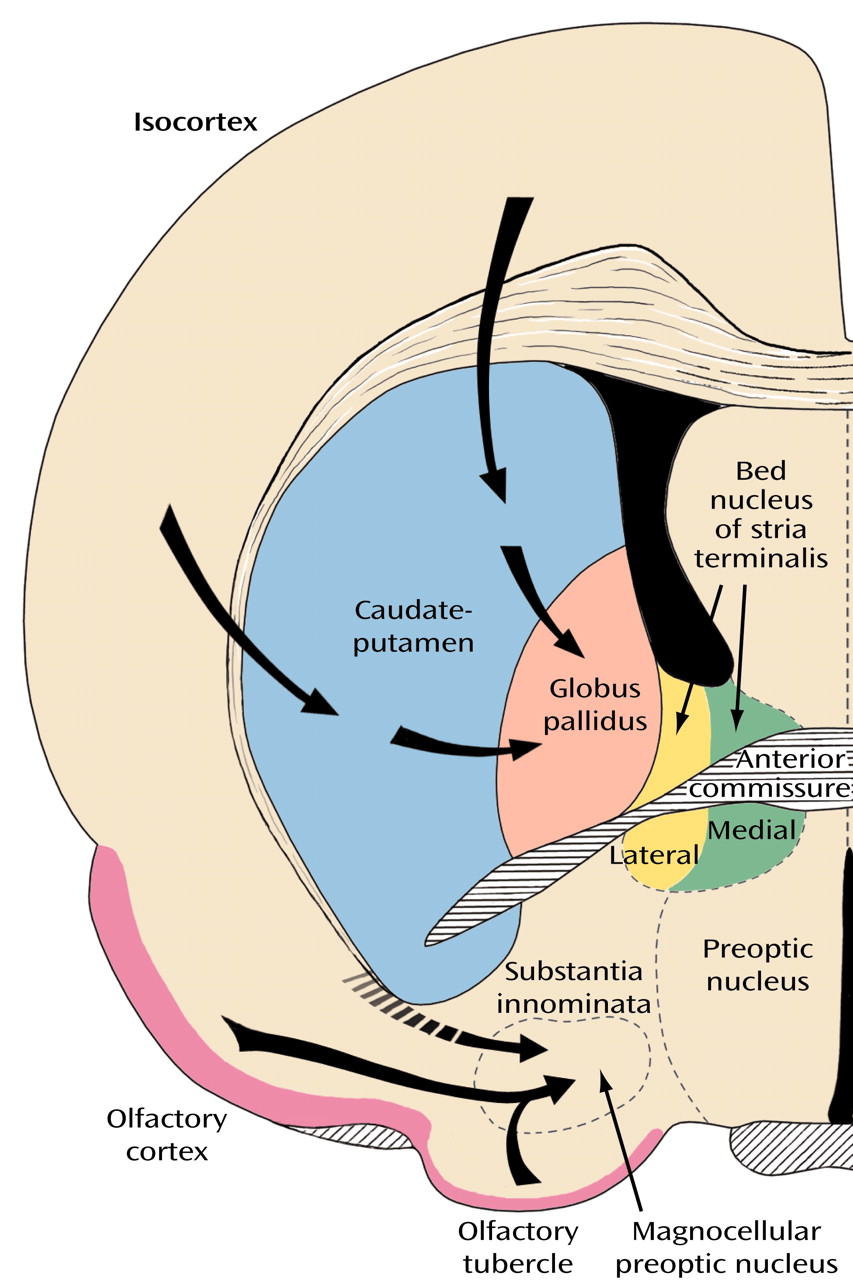
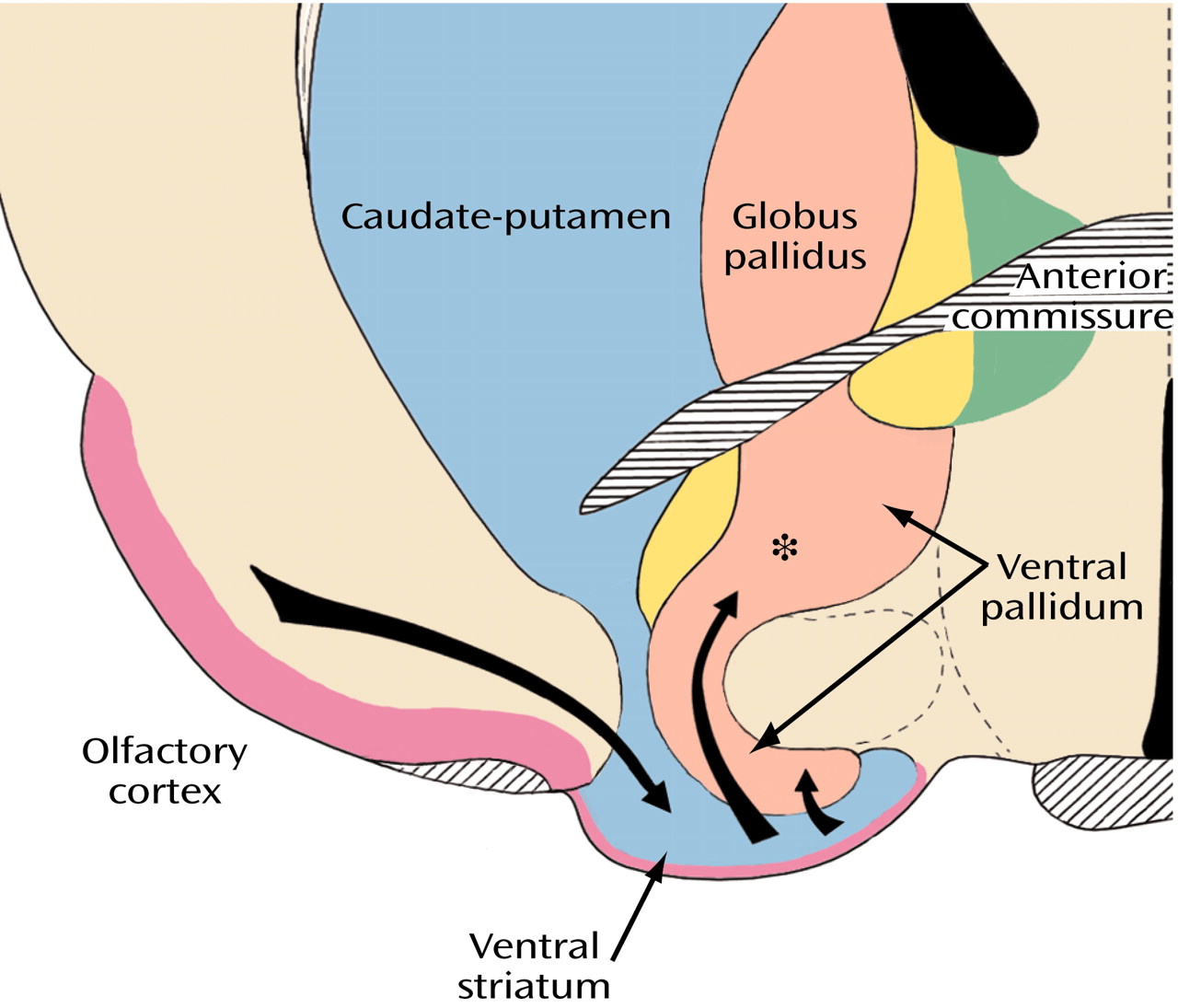
The broad striatal continuum (in blue) beneath the anterior section of the internal capsule (
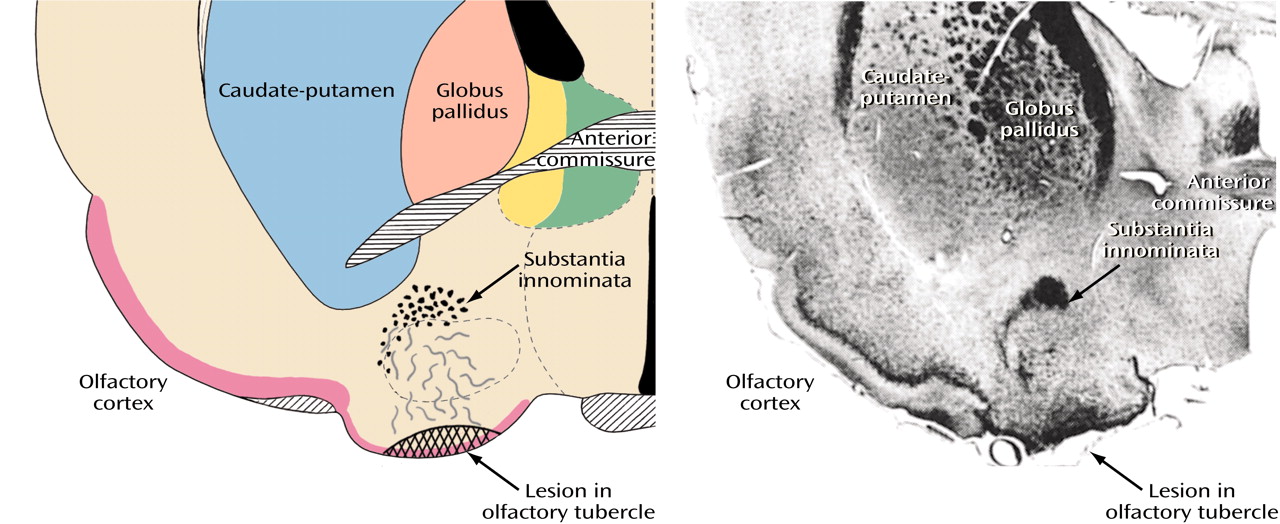
Figure 8, image A) includes the accumbens’ region of the ventral striatum, also referred to as the fundus striati, which is directly continuous with significant ventral striatal areas in what was previously referred to as the substantia innominata (
Figure 8, image B).
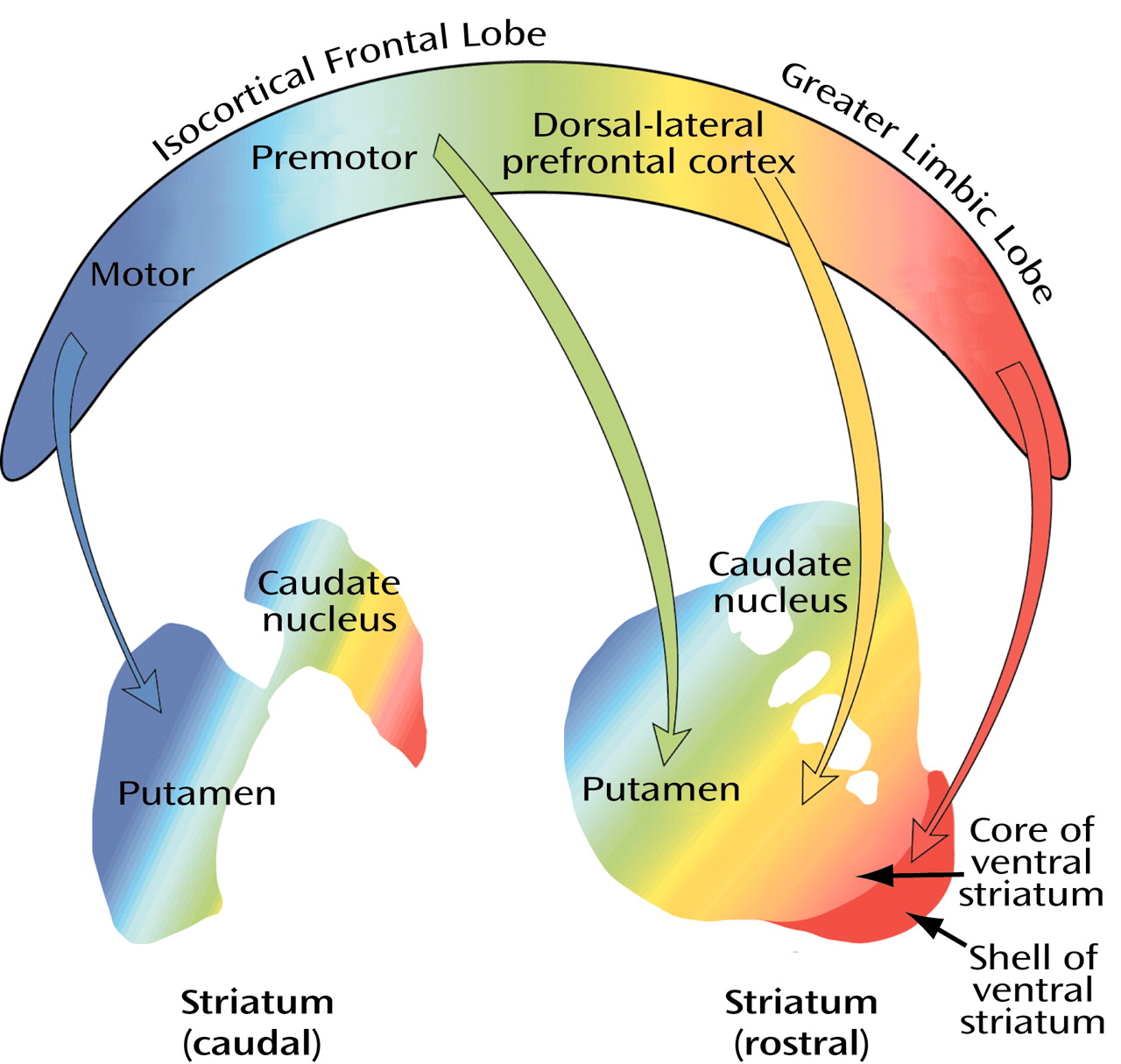
The subdivision of the striatal complex into ventral (orange), central (yellow), and dorsal (blue) territories (
Figure 9), which is based on the distribution of isocortical lobe and nonisocortical frontal limbic lobe projections to the striatum, serves as a good starting point for any discussion of cortical-subcortical reentrant circuits. Three circuits—the anterior cingulate, the lateral orbital-frontal, and the medial orbital-frontal—are related to the ventral emotional-motivational striatal domain (in orange). These circuits are closed in the sense that they supposedly originate and terminate in the same area of the frontal lobe. There is ample reason for uncertainty, however, since there seem to be a number of ways in which nearby cortical-subcortical reentrant circuits can interact. One intriguing scenario, mentioned earlier as a potential interface between the emotional-motivational domain and the motor system, is the spiraling of frontal-subcortical circuits from prefrontal areas related to the limbic lobe into the motor cortex
(44). The potential for translocation of information from one circuit to a nearby circuit is an important issue when one considers the current unwavering promotion of segregated frontal-subcortical circuits in the context of neuropsychiatric disorders.
In the wake of the great popularity of the theory of frontal subcortical circuits
(48), it may be tempting to overestimate their closed and segregated character, but their open character is equally impressive
(49). In other words, at every level of circuitry—cortical, striatal, pallidal, or thalamic—a variety of inputs from different parts of the brain have the potential to modify activity in the circuits. This point is particularly relevant regarding the ventral striatum, which receives a multitude of cortical projections from various regions of the greater limbic lobe
(50–
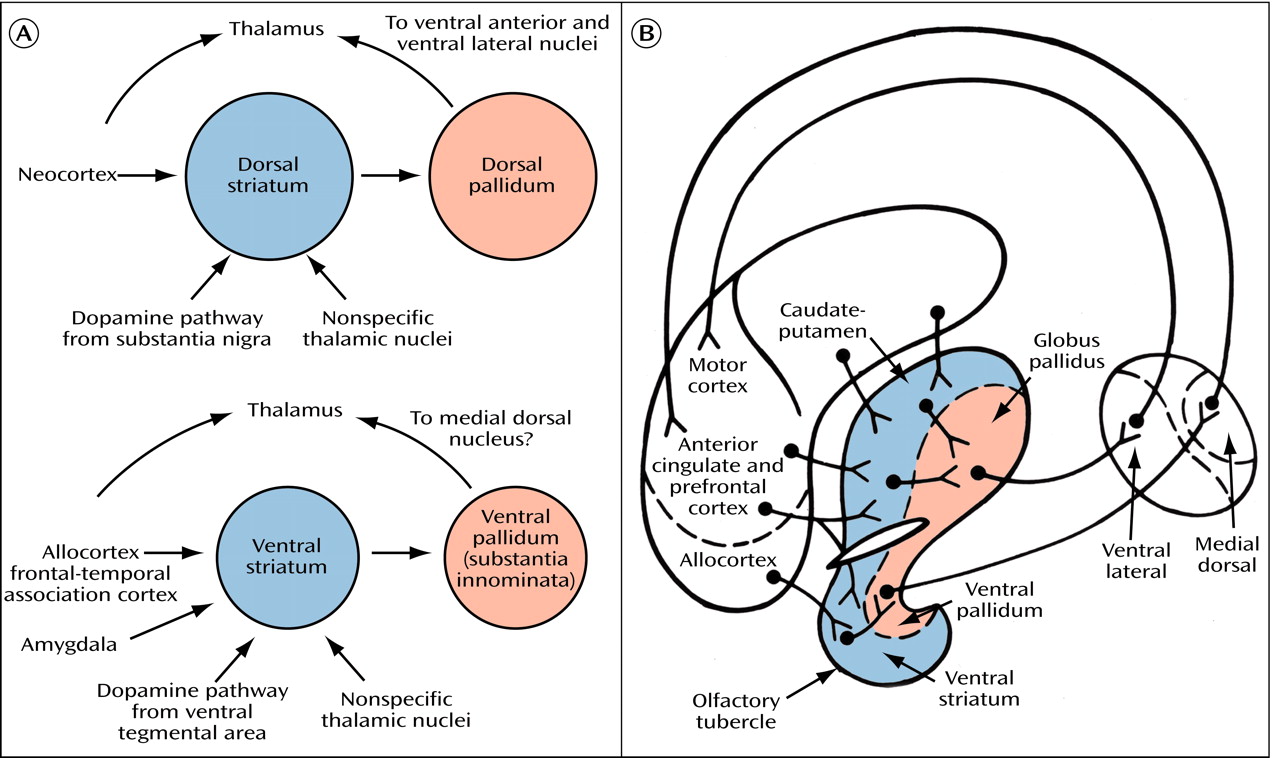
53). How the ventral striatum integrates these various cortical and subcortical inputs to adapt our behavior to changing environmental stimuli has been the focus of a large number of behavioral and pharmacological experiments. In a broad sense of functional subdivision, a consensus has gradually emerged that the prefrontal-subcortical reentrant circuit (with its various subcircuits) through the ventral striatal-pallidal system (the “motive” circuit of Kalivas et al.
[54]) is critical for the initiation and mobilization of appropriate adaptive behavior (the “reward-guided choice behavior” of Schultz et al.
[55]) in response to environmental stimuli similar to the way in which the motor loop through the dorsal parts of the basal ganglia is regarded as important for movement control.
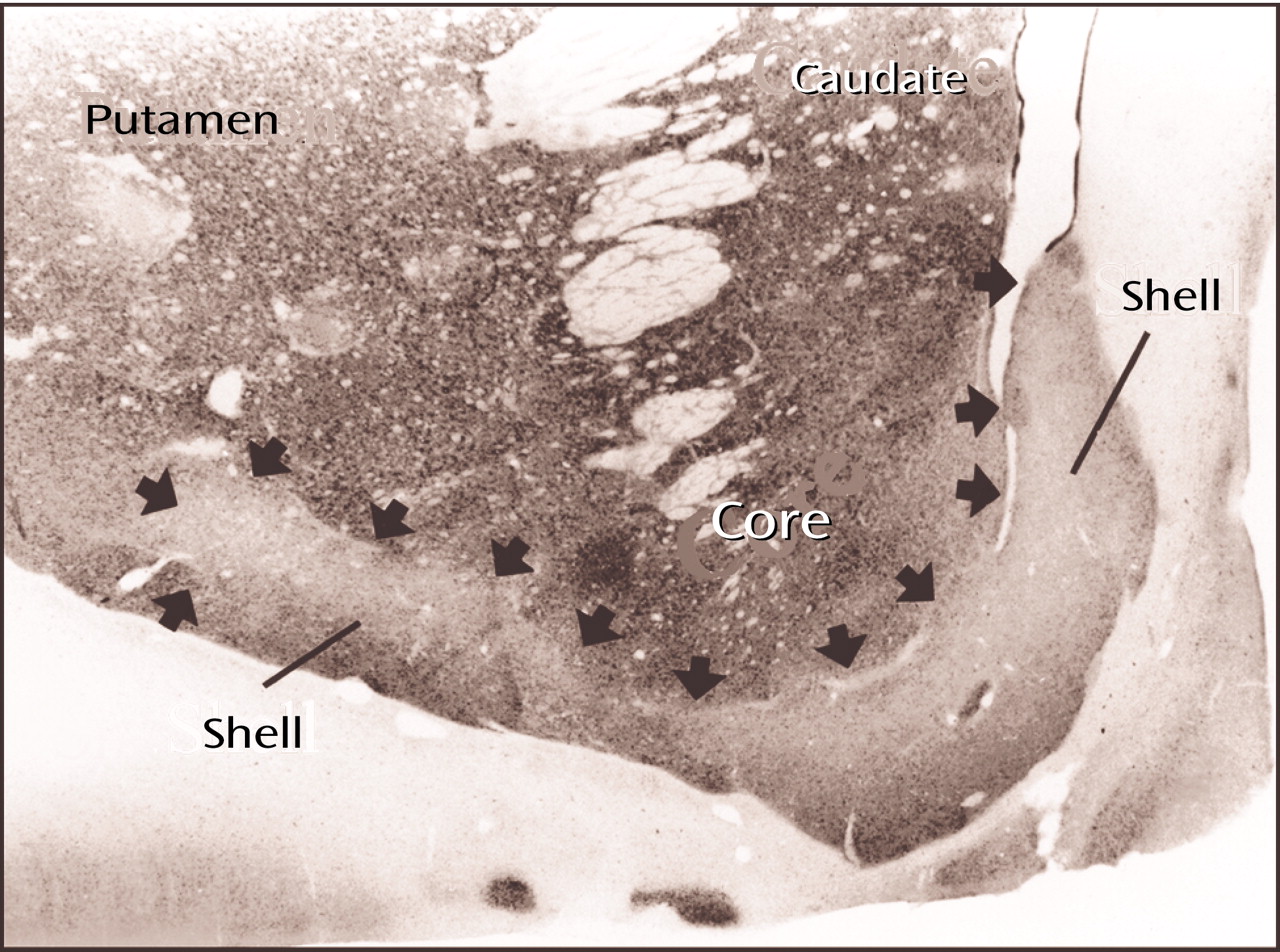
Core-Shell Dichotomy
With a number of different techniques, the peripheral, ventrally located shell of the ventral striatum (
Figure 10) can be distinguished from the central core, which merges imperceptibly with the rest of the striatum
(56). Although the shell, like the core, is part of the ventral striatum, it also has features that are reminiscent of the extended amygdala (
Figure 8, image A, indicated by the yellow areas) that translate into a number of anatomical and functional distinctions between the shell and the core
(57,
58). The shell is characterized by moderate to high opiate and dopamine D
1 and D
3 receptor binding, and the shell, rather than the core, seems to be an especially significant target for the action of antipsychotic drugs
(59). I mentioned earlier that the view of the accumbens as a specialized limbic-motor interface is deceptive. To refer to the nucleus accumbens in the sense of an anatomical entity is equally fallacious.
Small-Celled Islands
One of the most characteristic features of the ventral striatum is its small-celled islands
(60), which are especially prominent in the shell (
Figure 8, image A) but are present in other parts of the ventral striatum and also in the extended amygdala. Their neurons contain an abundance of opioid and dopamine receptors
(61) in addition to the Bcl-2 protein
(62), a marker of neuronal immaturity. When one considers the potential for development of these neurons, it is not difficult to envision the ventral striatum as especially prone to remodeling in response to changing circumstances. However, its greater reservoir of immature neurons may come with a price in the sense that the ventral striatum and extended amygdala may also be especially vulnerable to a number of damaging extrinsic and intrinsic factors throughout life, particularly during puberty
(63,
64).
Clinical Correlations
Ever since Swerdlow and Koob
(65) and Modell et al.
(66) used the cortical-subcortical reentrant circuit(s) through the ventral striatal-pallidal system in their discussions of depression, schizophrenia, and obsessive-compulsive disorders, an increasing number of authors have focused their attention on the functional and clinical correlates of the cortical-subcortical reentrant circuits through the ventral striatal-pallidal system. Not surprisingly, imaging studies of mood and affective disorders
(67,
68), obsessive-compulsive disorders
(69), and substance abuse
(70,
71) have consistently shown changes in cortical areas of the greater limbic lobe, which is the source of most of the cortical input to the ventral striatal-pallidal system.
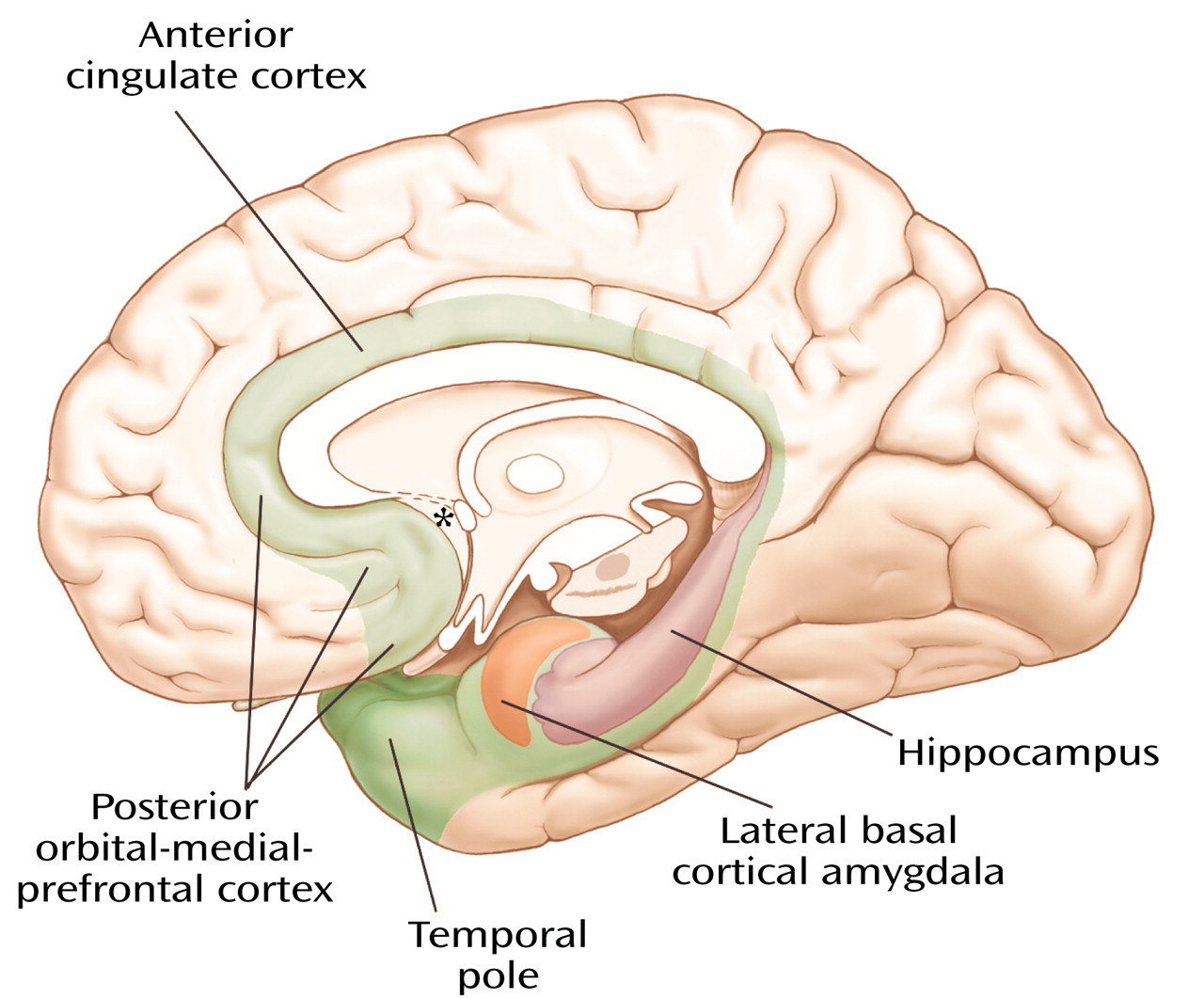
Although the dorsal-lateral prefrontal cortex has received much attention in schizophrenia, the greater limbic lobe areas and/or their circuits through the ventral striatal-pallidal system are likely to be of particular importance in the study of schizophrenia
(63,
72–74). Nevertheless, it is important to realize that the ventral striatal-pallidal system is only one of several prominent output channels of special significance for the phenomenology of schizophrenia and other neuropsychiatric disorders. The extended amygdala and the basal nucleus of Meynert, as well as the precommissural septum (see
Figure 6), are particularly important in this context since they, like the ventral striatal-pallidal system, receive cortical input primarily from the greater limbic lobe.
The Extended Amygdala
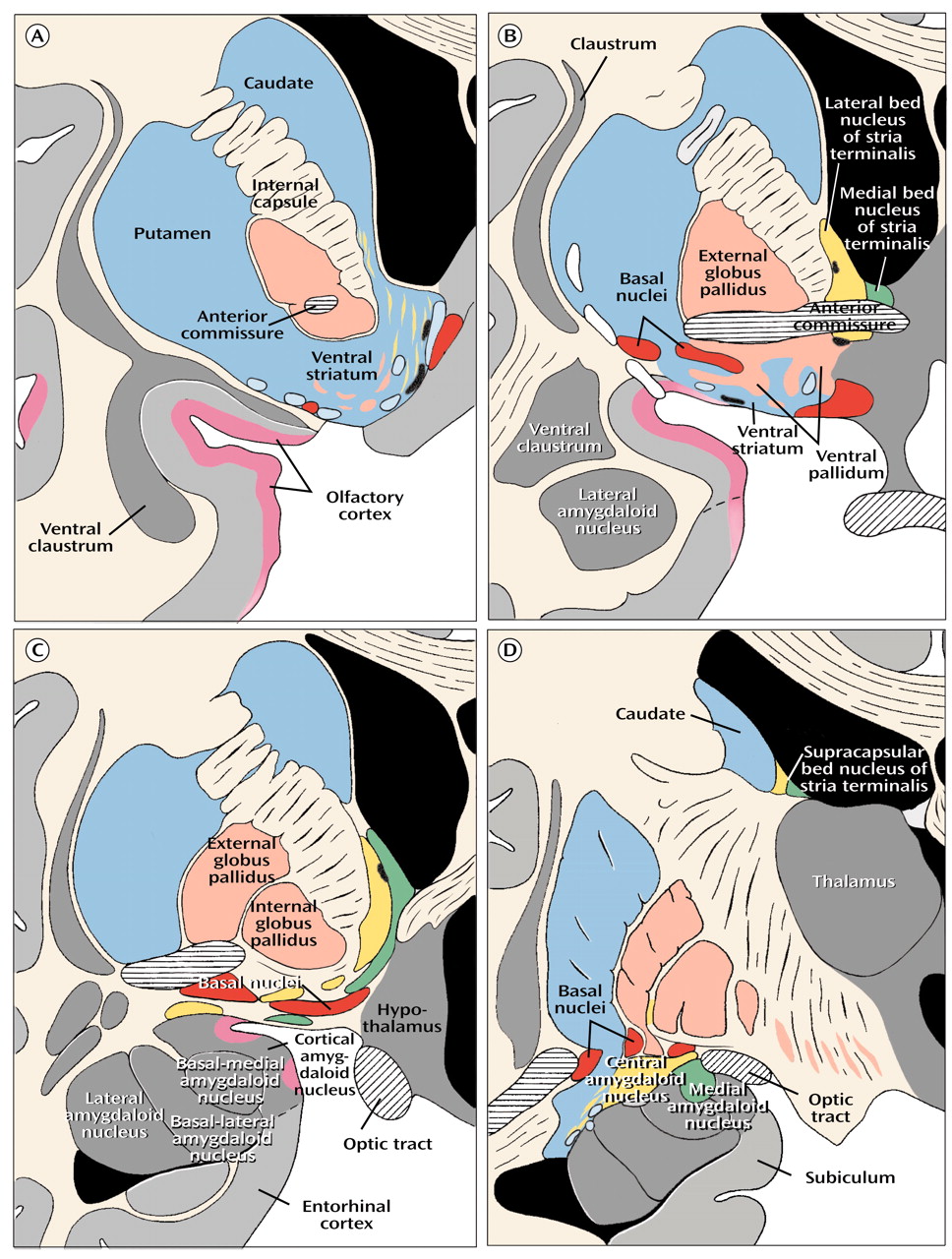
The notion that the bed nucleus of stria terminalis and the central medial amygdala form a continuous structure in human embryos and that remnants of this continuum can still be found in the form of interrupted cell columns within the stria terminalis as it makes a semicircular detour above and behind the internal capsule and thalamus to connect the bed nucleus (
Figure 8, image B) with the central-medial amygdala (
Figure 8, image D) was first suggested by Johnston in 1923
(8). Half a century later, José de Olmos
(9) identified a histochemically distinct subpallidal neuronal continuum between the central amygdaloid nucleus and the bed nucleus of stria terminalis in the adult rat. Although it is difficult, if not impossible, to illustrate the subpallidal part of the human extended amygdala as a continuum in sections that show only neuronal cell bodies (
Figure 8, image C), continuity is indicated by stains for peptidergic fibers and terminals that are typical for the bed nucleus of stria terminalis and the central-medial amygdala
(7,
47,
75,
76). In addition, far-reaching parallels between the two major components of the extended amygdala, i.e., the central-medial amygdala and the bed nucleus of stria terminalis, have been described, in regard to cytoarchitecture, histochemistry, and connectivity
(76,
77).
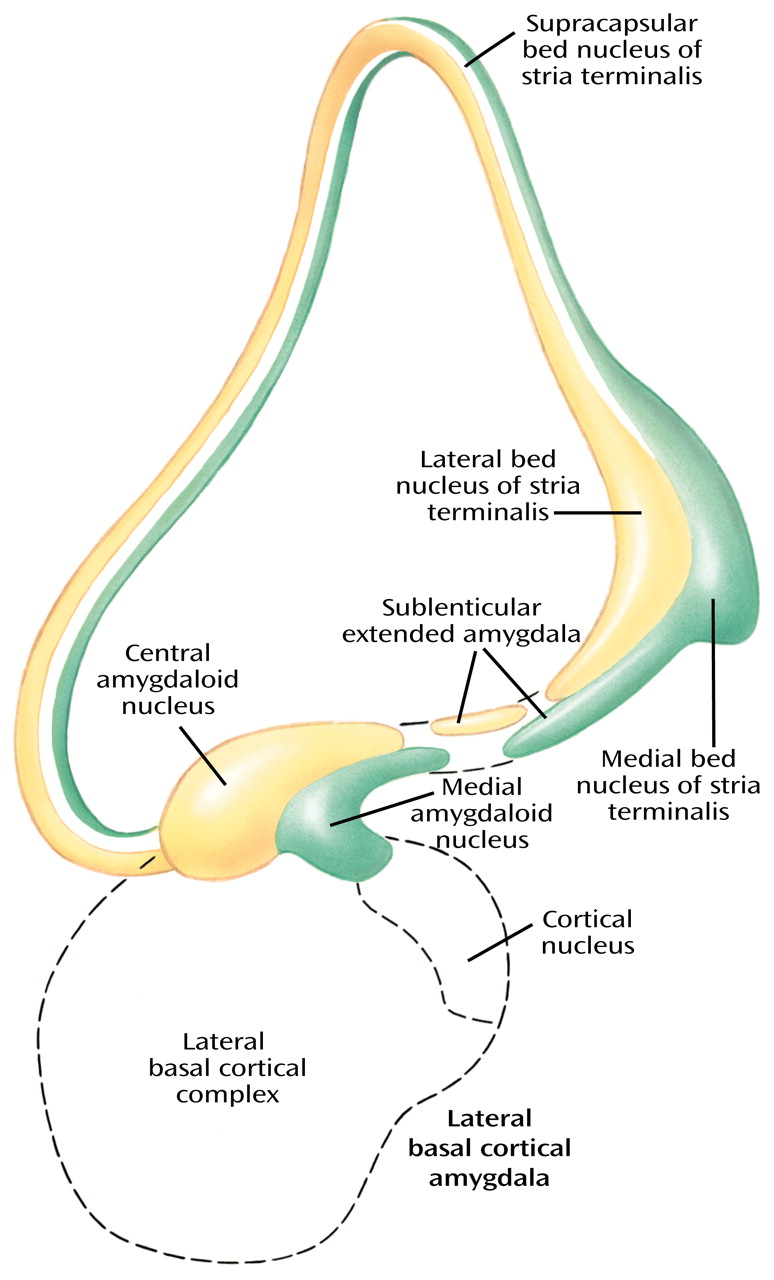
The extended amygdala (
Figure 11), which, as indicated earlier, does not include the lateral basal cortical amygdala, consists of central (yellow) and medial (green) divisions defined by their relations to central and medial amygdaloid nuclei, respectively. The extended amygdala is characterized by long and strikingly abundant associative connections and has prominent projections to autonomic and somatomotor centers in the lateral hypothalamus and brainstem (central division) and to the endocrine-related medial hypothalamus (medial division). The extended amygdala, like the ventral striatum, receives input primarily from nonisocortical regions of the greater limbic lobe, including the lateral basal cortical amygdala. Thus, it represents a strategically placed ring formation capable of coordinating activities in regions of the multiple limbic lobe forebrain for the development of coherent behavioral responses through the referenced output channels.
The prevailing view in the past has been that the bed nucleus of stria terminalis is ultimately a relay in a downstream projection from the central and medial amygdaloid nuclei. The concept of the extended amygdala, in which the bed nucleus and the central and medial amygdaloid nuclei are parts of the same macrostructure, represents a significantly different way of looking at this part of the brain. Not surprisingly, this paradigm shift has met with some resistance, as discussed elsewhere
(76,
77).
The question of whether the extended amygdala is a useful concept was debated during a discussion by Swanson and Alheid at a recent symposium
(78). According to Swanson, the central-medial amygdala represents a striatal structure for which the bed nucleus of stria terminalis serves as a pallidal counterpart. A larger number of studies, however, have corroborated Johnston’s original idea of a continuum involving the central-medial amygdala and the bed nucleus of stria terminalis
(8). Since anatomical and histochemical differences between this continuum and the striatal-pallidal system appear to be significant, we
(47,
76,
77) and others (e.g., reference
75) have argued that the continuum involving the central-medial amygdaloid bed and nucleus of stria terminalis should be identified as a macroanatomical system in its own right. Swanson thinks that these differences are not prominent and specific enough to justify the definition of the extended amygdala as a system separate from the striatal-pallidal system. As usual in situations in which differences of opinion are concerned, the proof will be shown in later findings. As indicated, a growing number of researchers have accepted the notion that the extended amygdala is a useful concept.
The extended amygdala has been accepted as a useful concept in investigations of drug addiction and other specific behaviors, ranging from fear and anxiety to sexual and appetitive behavior
(32), and it is slowly gaining a foothold in other fields related more directly to psychiatry
(79–
81). Much would be gained if scientists and clinicians interested in the amygdaloid complex would include the bed nucleus of stria terminalis (which is a large structure in the human brain) in their imaging studies of the amygdala.
There are a number of reasons to pay special attention to the extended amygdala in the context of neuropsychiatric disorders. Many receptors (e.g., for vasopressin, oxytocin, androgens) and neuropeptides (e.g., cholecystokinin, enkephalins, angiotensin II, somatostatin, neurotensin, opioid peptides) that have attracted special attention in the study of neuropsychiatric disorders
(82) are characteristic of one or both divisions of the extended amygdala. Androgen-receptive neurons, which are prevalent in the medial division of the extended amygdala, are of apparent importance for determining sexual behavior but are also well known for their involvement in aggressive behavior. Neurons that contain corticotrophin-releasing factor, which are especially prominent in the central division of the extended amygdala, are relevant in physiological responses to stress and for their apparent involvement in major depression and anxiety disorders. These and other features, which make the extended amygdala particularly important in the context of neuropsychiatric disorders, have been discussed in review articles
(7,
63,
82,
83). Since the extended amygdala, like the ventral striatum, contains an abundance of small-celled islands
(47), my earlier discussion regarding the small-celled islands is relevant also in regard to the extended amygdala.
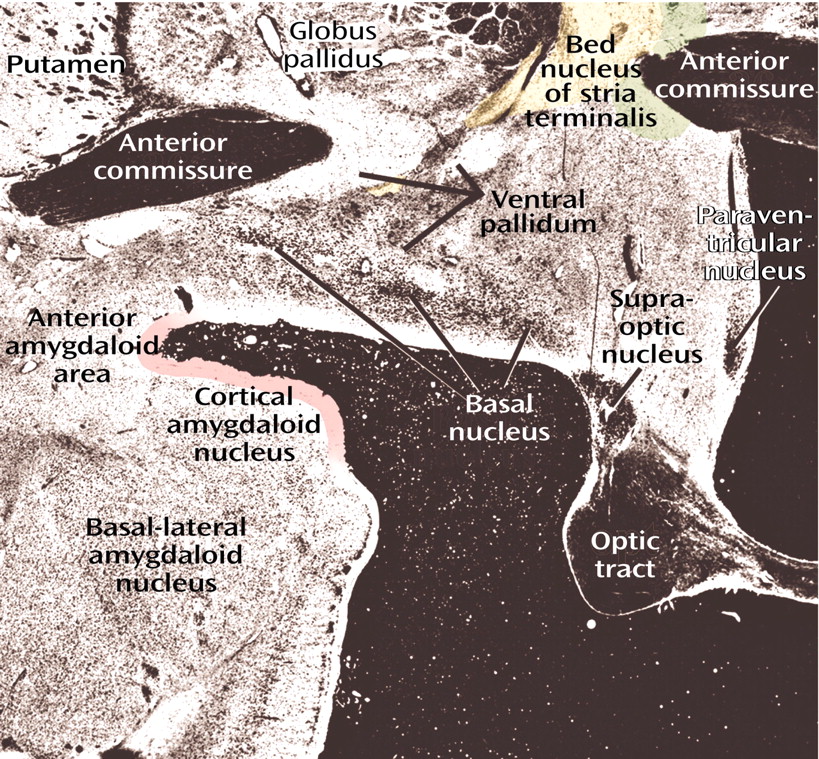
The Basal Nucleus of Meynert
The most conspicuous and best-known component of the basalis region is the basal nucleus of Meynert (colored red in
Figure 8, image B), which contains a mixture of cholinergic and γ-aminobutyric acid (GABA)-ergic cortical-petal neurons. This widely dispersed, more or less continuous collection of aggregated and nonaggregated neurons stretches from the septum-diagonal band in the rostral region of the forebrain to the caudal part of the globus pallidus. Since collections of the large hyperchromatic cells of this system are strikingly displayed in Nissl preparations (
Figure 12), the basal nucleus of Meynert has sometimes been used as a synonym for the substantia innominata or simply been referred to as the nucleus of the substantia innominata.
Cortical afferents to the basal nucleus come primarily from the greater limbic lobe, whereas basal nuclei projections, which are important for cortical arousal and attention mechanisms
(85), reach the entire cerebral cortex. These anatomical and physiological features signify the importance of the basal nucleus in the context of neuropsychiatric disorders
(86).
Although the most well-known clinical correlation in regard to the basal nucleus is Alzheimer’s disease, in which there is usually pronounced depletion of neurons in the basal nucleus, other degenerative disorders with frequent involvement of this nucleus include Parkinson’s disease, Korsakoff’s disease, and Down’s syndrome. It is important to realize, however, that these disorders also involve other parts of the brain. It hardly needs mentioning that the substantia nigra shows pronounced changes in Parkinson’s disease, whereas in Alzheimer’s disease, pathological changes in the hippocampus and parahippocampal gyrus are pathognomonic
(87). The hallmarks of Alzheimer’s disease also include paranoid delusions, hallucinations, and psychomotor agitation—symptoms commonly found in schizophrenia
(88). These symptoms, which apparently occur independent of the degree of dementia, can be correlated with pathological involvement of structures in the medial-temporal lobe, including the amygdala, which are characterized not only by connections with surrounding regions of the temporal lobe and the orbital-frontal cortex but also downstream projections to the ventral striatal-pallidal system, the extended amygdala, and the basal nucleus of Meynert.